Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 20
April 6th, 2018
CASE IV: 2012911922 (JPC 4032913).
Signalment: 11-year-old, female, spayed, Chihuahua (Canis familiaris), canine.
History: The dog was presented with a chief complaint of abdominal bloating. By abdominal ultrasonography, a large hepatic mass and hypoechoic lesion filling the peritoneal cavity were detected.
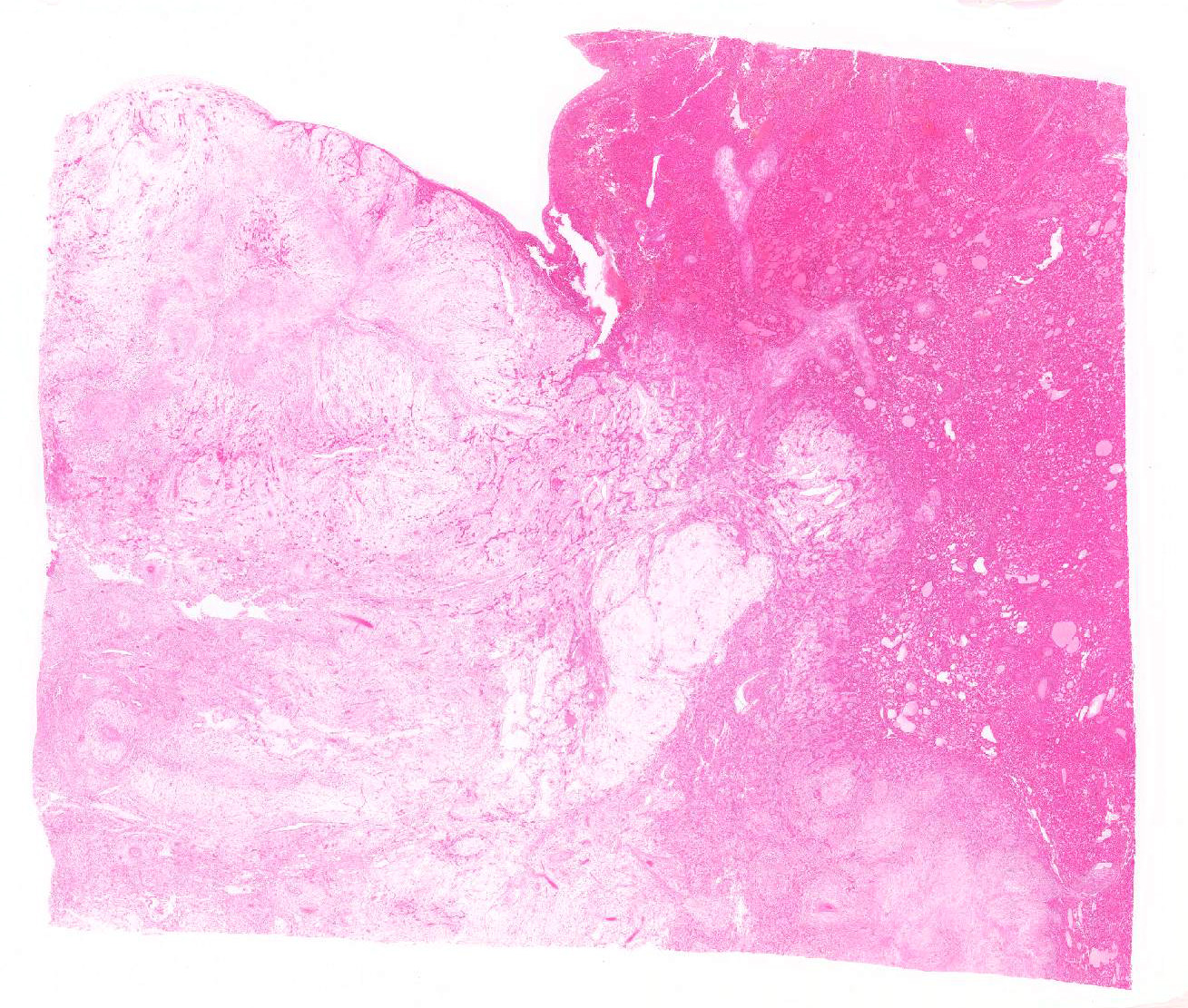
Gross Pathology: From surgical finding, the cystic mass originated from the hepatic left lateral lobe and filled the entire peritoneal cavity. The mass was the size of larger than 10 cm in diameters and was very soft and semitransparent milky to yellowish white mixed with blood. When made a cut in, a lot of mucus leaked, and the mass lost shape. The cut surface showed extensive myxomatous area. The site of liver attachment of the mass was harder and whiter than other area.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): None provided.
Microscopic Description:
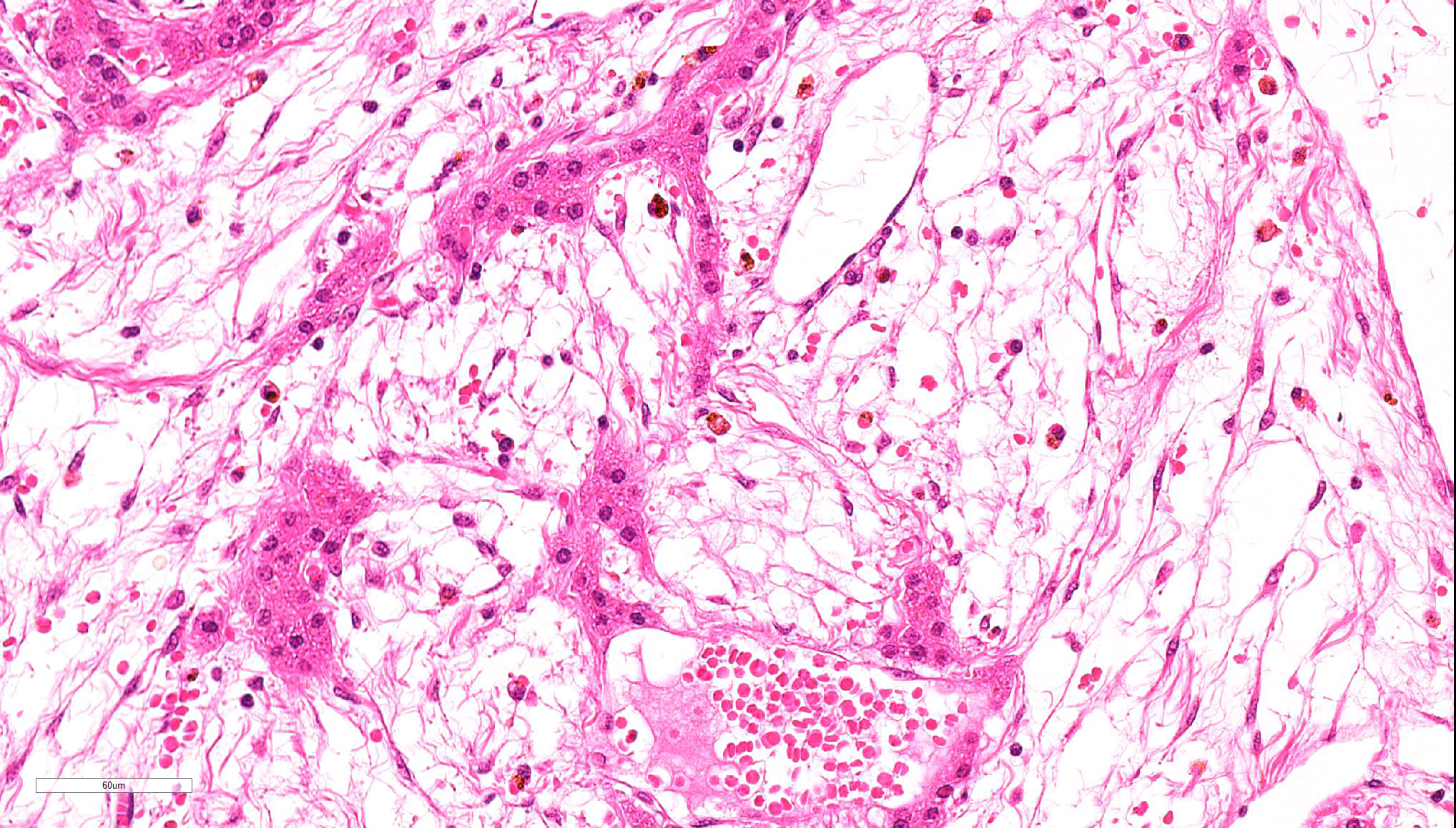
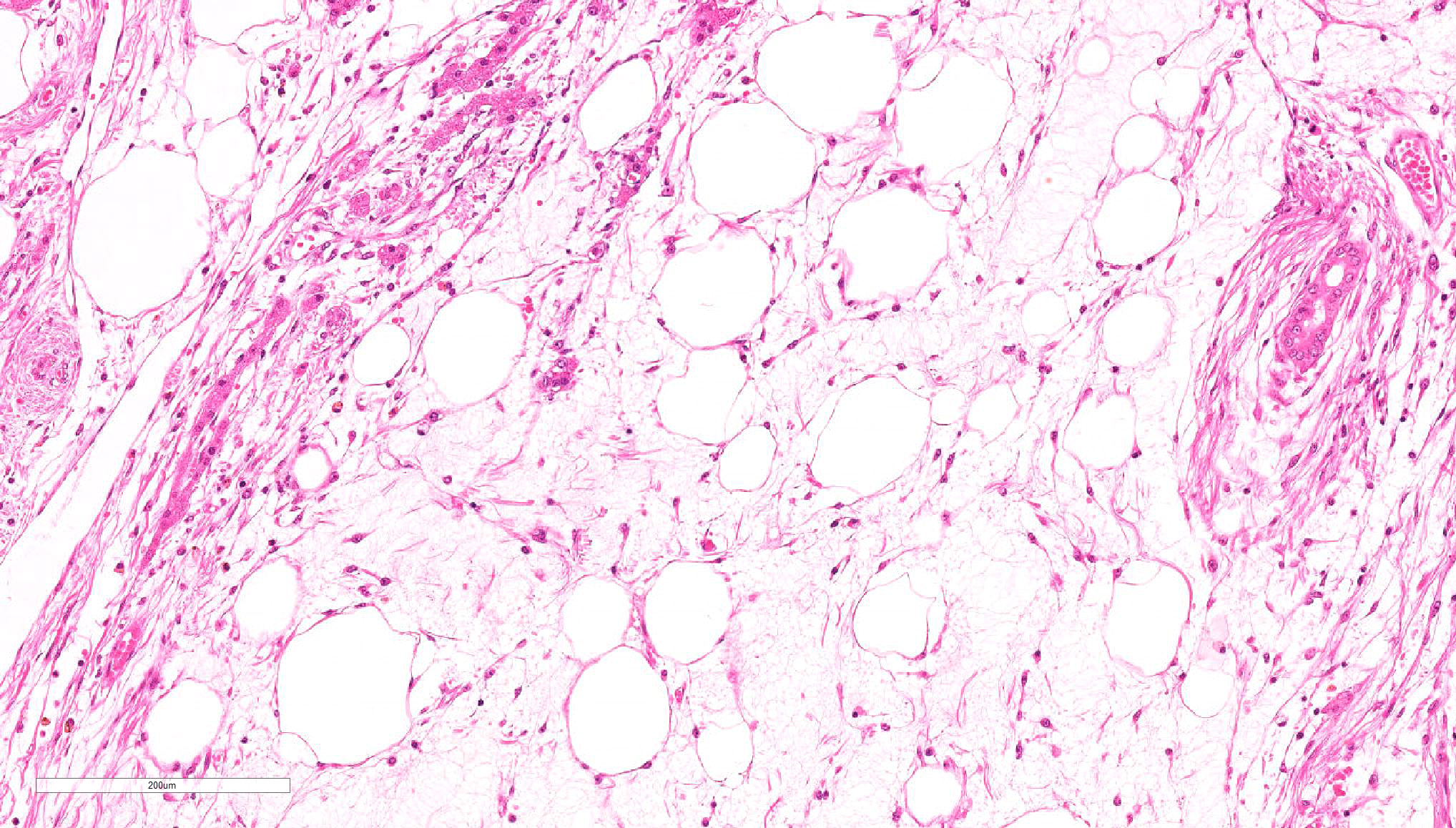
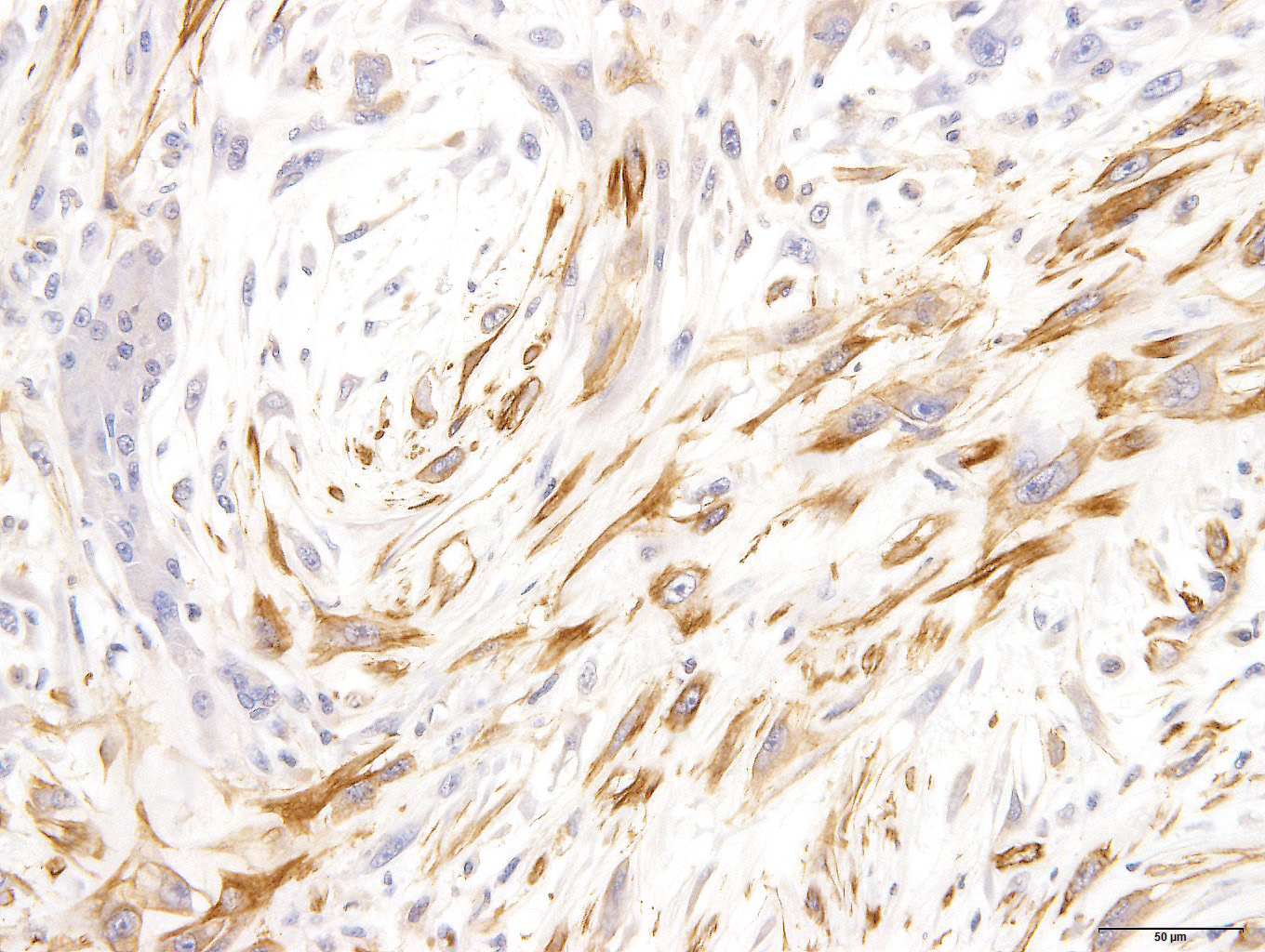
Spindle-shaped tumor cells chiefly proliferated in sheet or bundle with collagen fibers and mucinous matrix. Round or pleomorphic tumor cells also mingled with spindle-shaped tumor cells. The spindle-shaped tumor cells and collagen fiber were arranged in a concentric pattern in the perivascular areas. The tumor cells had round or oval nuclei of varying size with one or a few small to large distinct nucleoli. The cytoplasm of majority spindle shaped tumor cells was poor-marginated scant to abundant eosinophilic cytoplasm. Some pleomorphic to round tumor cells had several small or large vacuoles or a single giant vacuole like signet-ring-cell. Signet-ring shaped cells had usually thin cytoplasm compressed by a large vacuole and peripherally located nuclei. A few multinucleated tumor cells and large round cells were also observed. Mitotic figures were often seen. Hepatic cords and bile ducts were often remained between tumor cells within the mass, and the bile ducts were showed mild to moderate reactive hyperplasia. Mild hemorrhage and inflammatory cell infiltration were also seen. In the peripheral area of mass, thin spindle-shaped tumor cells proliferated with amounts of collagen fibers and invaded between hepatic cords.
On a Masson’s trichrome stain, spindle-shaped tumor cells and collagen fibers were stained weak blue in contrast with dark blue at the edges of hepatic cords. However, vacuolated round tumor cells were unstained.
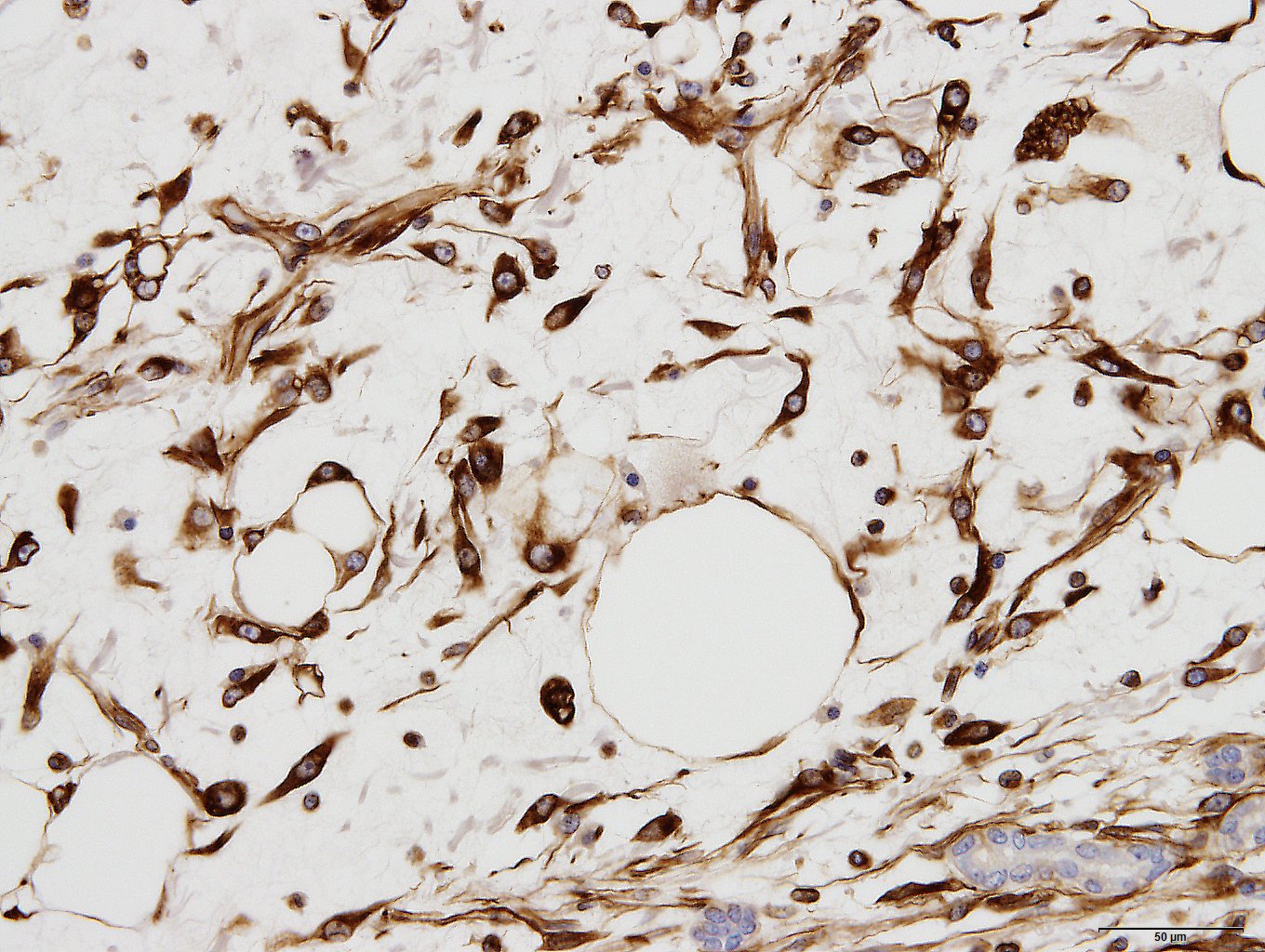
Immunohistochemically, spindle-shaped and vacuolated round tumor cells were strongly to moderately positive for vimentin. The spindle-shaped tumor cells were variably positive for alpha-smooth muscle actin. But all tumor cells were negative for desmin. Tumor cells of both types were intracellular strongly positive for laminin. The extracellular matrix was often weakly positive for vimentin, alpha-smooth muscle actin and laminin.
Contributor’s Morphologic Diagnosis:
Liver: Ito cell tumor, malignant
Contributor’s Comment: Mesenchymal tumors in liver are rare in human and animals. Hemangiosarcoma and leiomyosarcoma appear more commonly in animals.7,12
The normal liver tissue consists of four types of sinusoidal lining cells; endothelial, Kupffer, pit and hepatic stellate (Ito) cells. Stellate (Ito) cell also called perisinusoidal cell, vitamin A-storing cells, fat-storing cells, lipocytes, and interstitial cells. It is difficult to distinguish between each cell types by the routine light microscopy, so immunohistochemical stains and ultrastructural analysis are helpful.
Stellate (Ito) cells contain lipid droplets in cytoplasm, store vitamin A in lipid droplets, and play a role in the storage and regulation of vitamin A.8 These cells have also another major role to produce extracellular matrix proteins.11 During the proliferating process, stellate (Ito) cells lost fat droplets and vitamin A with myofibroblast-like appearance, and produce great amounts of extracellular matrix.8,11 In immunohistochemical stains, stellate (Ito) cells without fat droplets and vitamin A are positive for desmin and actin/alpha-smooth muscle actin. It supports to identify proliferating stellate (Ito) cells as cells have myofibroblastic function.4,8,11
Present tumor was consisted of two principal histological types of tumor cells: spindle-shaped and vacuolated round. Those tumor cells proliferate within remaining hepatic cords and bile ducts and invaded hepatic parenchyma, producing collagen fibers. It seemed that this tumor arose from the hepatic sinusoid.
Similar proliferative lesions have been reported rarely in animals1,4,10,14,15 and humans.13 These reports concluded or suggested stellate (Ito) cell origin from not only morphohistochemical features but also immunohistochemical and ultrastructural features.1,4,10,15 Tumor cells in previous reports were immunoreactive for vimentin, desmin, α-smooth muscle actin, laminin, tenascin, and alpha B-crystallin. Tumor cells of present tumor show immunoreaction similar to previous reports with the exception desmin, suggesting that the present tumor originated from stellate (Ito) cells. Those previous reports suspected hepatic stellate (Ito) cell tumor was benign.1,4,10,15 However, our present tumor had cellular atypia and many mitotic figures suggesting a malignant tumor.
Tumors suspected hepatic stellate (Ito) cell origin were diagnosed by use of various diagnostic term1,4,10,13,15 because stellate cell had a lot of synonym. We diagnosed present tumor as malignant Ito cell tumor by references from the discussion of Stroebel P et al.15
Myxoid liposarcoma should be considered in the differential diagnosis.17 Some tumor cells of present case had small to large fat vacuoles in their cytoplasm. These tumor cells were suspended individually in a myxoid matrix similar to that seen in myxoid liposarcoma. However, the immunohistochemical profile including smooth muscle actin and laminin was different from our present case. Especially, laminin is a major compound of the hepatic extracellar matrix. In the repair processes of focal hepatic injury, laminin positive interstitial matrix are increased with Ito cells proliferation after infiltration of various inflammation cells.12 Intra- and extra- cellular laminin expression were seen in the previous Ito cell origin tumors.10,16 In addition, hepatic cords often remained in the tumor tissues. It seems that the tumor arise between hepatic cords and proliferated with separation of them. The histomorphologic and immunohistochemical characteristics supported that present tumor originated from Ito cells.
JPC Diagnosis: Liver: Sarcoma, poorly differentiated, with myxoid differentiation, Chihuahua (Canis familiaris), canine.
Conference Comment: Hepatic stellate (Ito) cells are remarkably diverse mesenchymal cells found between hepatocytes in the space of Disse and serve primarily as storage cells for lipid and vitamin A in large round vacuoles. The functionality of stellate cells does not end there, they also play a role in the following arenas: hepatic fibrosis, cytokine release, blood flow, and antigen presentation.5
When the liver is damaged, stellate cells become activated and take on a myofibroblastic phenotype, evidenced by the presence of immunohistochemically measurable α-smooth muscle actin, with concomitant loss of lipid vacuoles. In addition to the production of collagen and other extracellular matrix components (proteoglycans, fibronectin, and hyaluronin) activated stellate cells also release cytokines which are proinflammatory, profibrogenic, and promitotic.5
Stellate cells can affect sinusoidal blood flow by producing matrix and narrowing sinoisoidal lumina and by constriction of myofibroblastic stellate cells around endothelial cells. This act of constriction is induced by increased production of endothelin-1 by sinusoidal endothelial cells, which, in health, is balanced by vasodilation stimulated by nitric oxide also released by sinusoidal endothelial cells.5
Finally, stellate cells act as antigen-presenting cells in the liver much the same as Kupffer cells. In this realm, they mostly function to present lipid antigens to CD1-restricted T lymphocytes (like natural killer T-cells), but also present antigenic peptides to CD4+ and CD8+ T-cells with priming of CD8+ T-cells.18
Stellate (Ito) cell tumors are more common in mice than other domestic animals but are still rare.3 Gross lesions are described as moderately firm, pale white nodules found within the liver parenchyma. Microscopically the nodules are composed of nodular aggregates of round to spindle cells that have dark oval nuclei and variably sized clear cytoplasmic vacuoles on a myxomatous matrix. These vacuoles are positive for oil red O and Sudan black staining, identifying them as lipid. Tumor cells are positive for vimentin, actin, desmin, and proliferating cell nuclear antigen. Additionally, the extracellular matrix stains positive for laminin and tenascin.16 A key differential in rodents is Ito cell hyperplasia which is described associated with Helicobacter sp. infection and numerous other circumstances. The main difference is with hyperplasia there is usually concurrent Kupffer cell hyperplasia as well.3
The conference participants as well as the moderate (an internationally renowned hepatic specialist) had difficulty precisely diagnosing the neoplasm as well as identifying it as of Ito cell origin. The moderator first cited the gross findings which state that mucus leaked out of the mass when it was incised; in his experience, stellate cell tumors rarely have that much myxomatous material present. To further underscore the difficulty in attributing the origin to that of Ito cells, the moderator cited several articles. The first one discussed morphologic characterization of myofibroblastic cells in the liver – the portal myofibroblasts and stellate cells.9 They found that desmin was unreliable in differentiating between the two, but that stellate cells were generally negative for vimentin. This fact is in stark contrast with the present case, in which the neoplastic cells are vimentin-positive. Further, the moderator elaborated on the presence of abundant mucin grossly which he stated may be more representative of a hemangiopericytoma, which like many types of mesenchymal neoplasma may demostrate a myxoid variant that is desmin negative (similar to this case).2 Finally, mesenchymal hamartomas may have a similar appearance to this neoplasm with a loose stroma surrounding ducts. Mesenchymal hamartomas are developmental anomalies in the biliary system of horses and have also been reported in humans. Considering all facts, the moderator would prefer sarcoma (myxoid variant) rather than being specific about cell type.
In an effort to be as specific as possible, a battery of histochemical and immunohistochemical stains were run including: Alcian blue, S-100, GFAP, smooth muscle actin, and desmin. The myxomatous matrix within the neoplasm is Alcian blue-positive but all other stains are negative. including smooth muscle actin, but which was positive for the contributor. In our stain, there is good internal control with strong intracytoplasmic immunoreactivity of the smooth muscle cells in vessels and myoepithelial stellate cells within portal regions, but the neoplastic cells are diffusely negative. Furthermore, we consulted M.D. gastrointestinal pathology subspecialists who admittedly had not seen an Ito cell tumor and could find no report of such a neoplasm in the human literature. In their experience, this is consistent with a myxoid fibrosarcoma, but had poorly differentiated liposarcoma as a differential due to the presence of fat.
Contributing Institution:Department of Pathology
Faculty of Pharmaceutical Sciences
Setsunan University
45-1 Nagaotohge-cho
Hirakata, Osaka 573-0101, Japan
http://www.setsunan.ac.jp/~p-byori/
References:
- Adkison DL, Sundberg JP. "Lipomatous" hamartomas and choristomas in inbred laboratory mice. Vet Pathol. 1991;28: 305-312.
- Avallone G, Helmbold P, Caniatti M, Stefanello D, Nayak RC, Roccabianca P. The spectrum of canine cutaneous perivascular wall tumors: morphologic, phenotypic, and clinical characterization. Vet Pathol. 2007;44(5):607-620.
- Barthold SW, Griffey SM, Percy DH. Mouse. In: Pathology of Laboratory Rodents and Rabbits. 4th Ames, IA: John Wiley & Sons, Inc.; 2016:101,114.
- Carpino G, Morini S, Ginanni Corradini S, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37: 349-356.
- Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 2. 6th ed. St. Louis, MO: Elsevier; 2016:288-289.
- Dixon D, Yoshitomi K, Boorman GA, et al. "Lipomatous" lesions of unknown cellular origin in the liver of B6C3F1 mice. Vet Pathol. 1994;31: 173-182.
- Head K CJ, Dubielzig R, Else R, et al. Histological Classification of Tumors of the American System of Domestic Animals. 2nd ed. Vol. X. Washington, DC: Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology and the Worldwide Reference on Comparative Oncology; 2003:119-133.
- Higashi N, Sato M, Kojima N, et al. Vitamin A storage in hepatic stellate cells in the regenerating rat liver: with special reference to zonal heterogeneity. Anat Rec A Discov Mol Cell Evol Biol. 2005;286: 899-907.
- Ijzer J, Roskams T, Molenbeek RF, Ultee T, et al. Morphological characterization of portal myofibroblasts and hepatic stellate cells in the normal dog liver. Comparative Hepatology. 2006; 5:7-15.
- Mohr U. International classification of rodent tumors the mouse. Hannover: WHO International Agency for Research on Cancer; 2001: 76-77.
- Nelson V, Fernandes NF, Woolf GM, et al. Primary liposarcoma of the liver: a case report and review of literature. Arch Pathol Lab Med. 2001; 125: 410-412.
- Ogawa K, Suzuki J, Mukai H, et al. Sequential changes of extracellular matrix and proliferation of Ito cells with enhanced expression of desmin and actin in focal hepatic injury. Am J Pathol. 1986;125: 611-619.
- Patnaik AK, Hurvitz AI, Lieberman PH. Canine hepatic neoplasms: a clinicopathologic study. Vet Pathol. 1980; 17: 553-564.
- Shintaku M, Watanabe K. Mesenchymal hamartoma of the liver: a proliferative lesion of possible hepatic stellate cell (Ito cell) origin. Pathol Res Pract. 2010; 206: 532-536.
- Stroebel P, Mayer F, Zerban H, et al. Spongiotic pericytoma: a benign neoplasm deriving from the perisinusoidal (Ito) cells in rat liver. Am J Pathol. 1995;146: 903-913.
- Tillmann T, Kamino K, Dasenbrock C, et al. Ito cell tumor: immunohistochemical investigations of a rare lesion in the liver of mice. Toxicol Pathol. 1999; 27: 364-369.
- Weiss SW GJ. Soft Tissue Tumors. 5th ed. St. Louis, MO: Mosby; 2008: 477-516.
- Winau F, Hegasy G, Weiskirchen R, Weber S, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. 2007;26(1):9-10.