Signalment:
Gross Description:
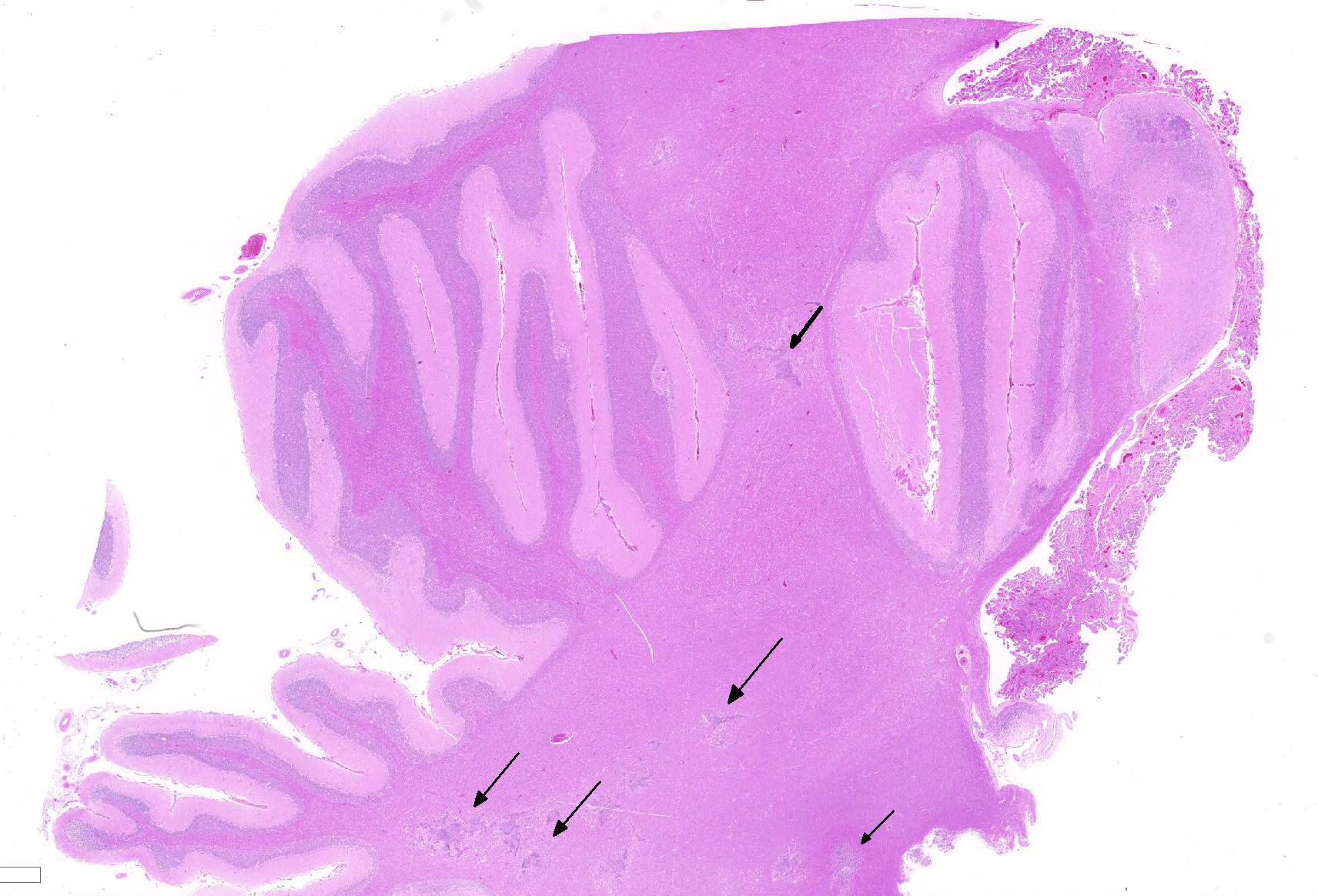
Histopathologic Description:
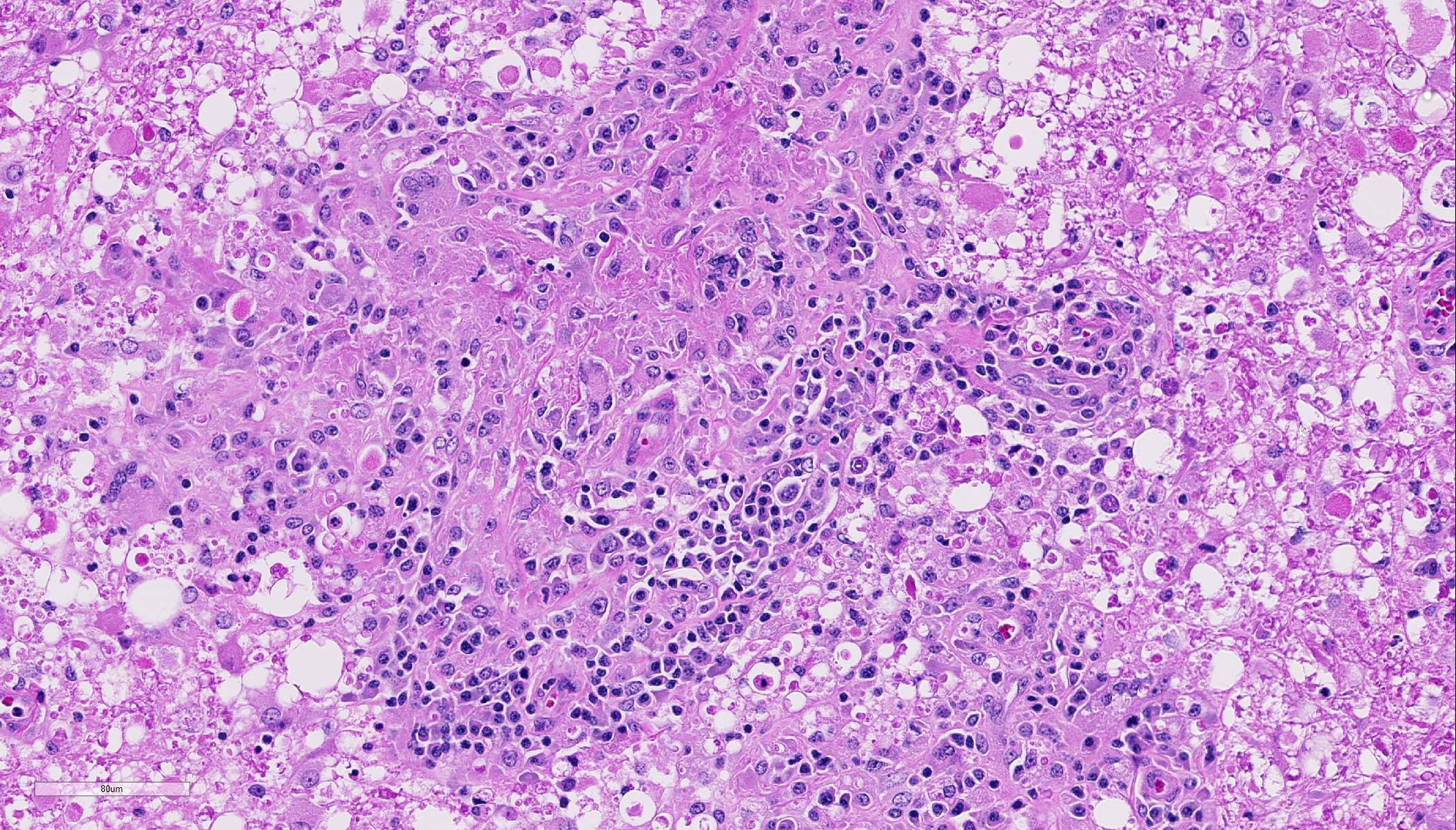
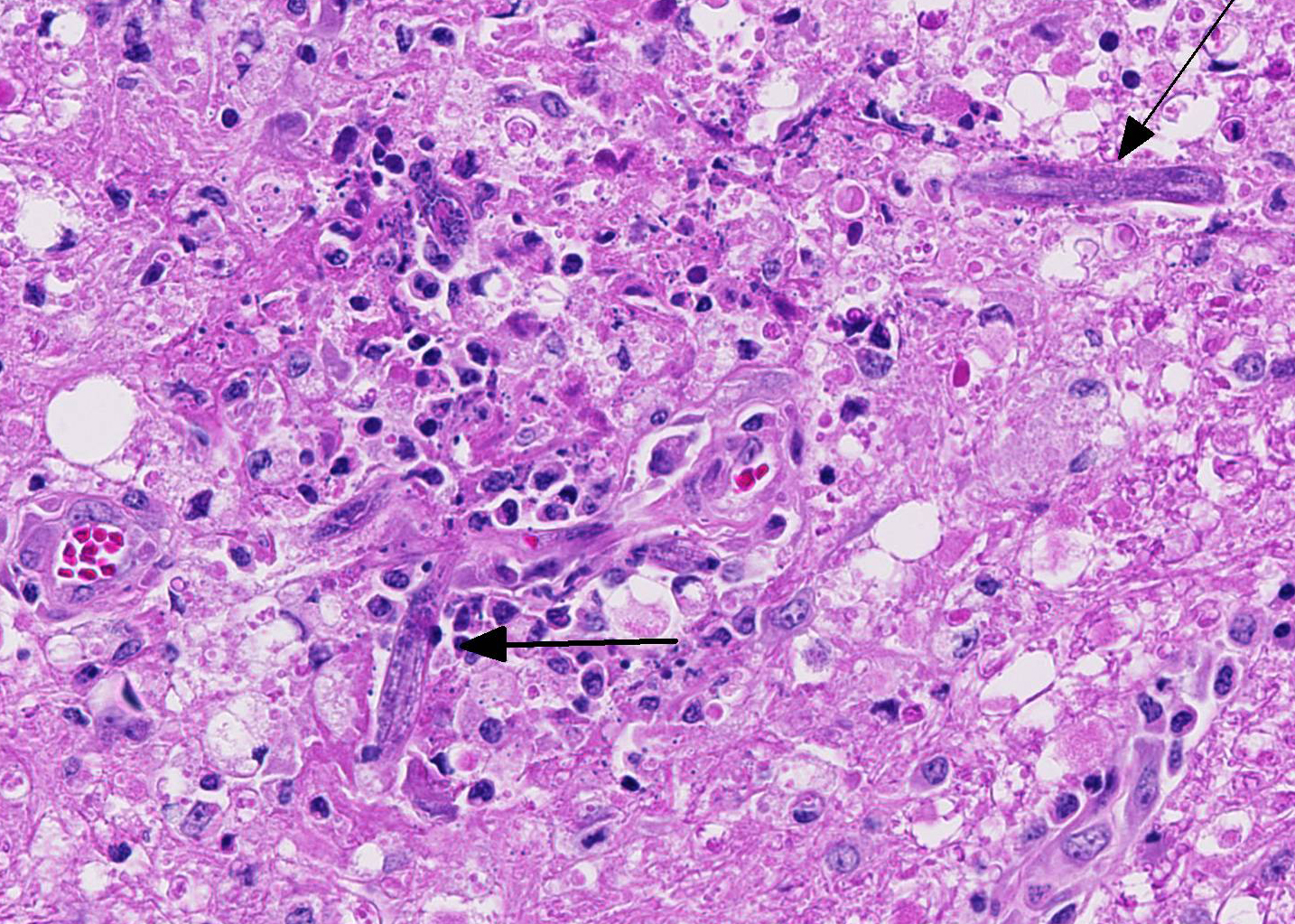
Within the cerebellar nuclei, there are multifocal, randomly distributed areas of necrosis, characterized by rarefaction of the neuropil, loss of architecture, and small amounts of eosinophilic cellular and karyorrhectic debris, admixed with several sections of nematodes and moderate numbers of gitter cells. Multifocally, perivascular infiltrates of macrophages, multinucleated giant cells, lymphocytes and plasma cells admixed with occasional nematodes expand the Virchow-Robin spaces and extend within the adjacent neuropil. Adjacent to the perivascular cuffs, there is marked vacuolation of the neuropil, dilated myelin sheaths and multiple round eosinophilic bodies (axonal spheroids). The meninges are multifocally infiltrated with low numbers of lymphocytes and macrophages.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
A non-suppurative inflammation, similar to viral infections, is usually encountered in infections. Equine protozoal myeloencephalitis caused by Sarcocystis neurona (and much less commonly S. hughesi) occurs in North America in the geographical range of opposums (Didelphis spp.), as the latter represent the definitive host for S. neurona. The lesions with Sarcocystis infection more typically affect the cervical and thoracic spinal cord, but lesions in the brain stem can occur. S neurona schizonts are oval or irregularly round, have very thin walls and contain few basophilic ovoid merozoites.4
Horses are considered one of the less sensitive species to the pathogenic effect of Toxoplasma gondii, as a specific pathologic condition related to toxoplasmic infection has not been described under natural conditions or experimentally.14 Granulomatous amebic encephalitis, caused by Balamuthia mandrillairs infection, occurs worldwide, albeit rarely, and has been described in horses.10 Amebae resemble large macrophages with foamy cytoplasm, large eccentric nucleus but can be distinguished by positive staining with PAS.
Trypanosoma evansi infection was reported to cause necrotizing encephalitis in Brazilian horses, with the most severe lesions occurring within the cerebral white matter.12 The larvae of tapeworms of the family Taeniidae, such as Coenurus cerebralis (larval stage of Taenia multiceps), although rarely affecting horses, can migrate and reach various organs, in particular the liver and the CNS, where they form cysts of varying size, which can be sometimes very large. In acute helminth infections, the inflammatory pattern is typically suppurative in nature with variable numbers of eosinophils, while chronic lesions develop a granulomatous component. Rare cases of cerebrospinal nematodiasis in horse have been attributed to Strongylus vulgaris.3 Angiostrongylus cantonensis has been reported to induce neurological disease in foals.15 Parelaphostrongylus tenuis, commonly called meningeal worm or brain worm, is a common neurotropic parasitic nematode of white-tailed deer in eastern North America; however a recent report revealed that horses are also susceptible to infection, and developed lesions mostly within the cerebellar parenchyma and associated meninges.13 The lesions within the CNS result from aberrant nematode larval migration, inducing areas of malacia (mostly in the white matter) with swollen axons and hemorrhages. The migration tracks induce reactive changes including vascular proliferation, invasion of macrophages, and perivascular cuffing containing varying numbers of eosinophils.
Similar lesions, caused by larval wandering of the microfilaria Setaria sp. in the brain and spinal cord are described in horses (aberrant hosts) in Asia. Setaria digitata is normally found as an adult in the peritoneal cavity of cattle and buffalo.8 Intracranial invasion by the nematodes Halicephalobus gingivalis is reported in horses in Europe, North and South America. Depending on the area of the CNS affected, lesions are granulomatous and eosinophilic meningoencephalitis, myelitis, poly-radiculitis or even cauda equina neuritis-like lesions. Parasitic granulomas in the kidney and gingiva may accompany the cerebral invasion.1 Halicephalobus gingivalis has a characteristic rhabditiform esophagus, composed of a corpus, isthmus and bulb. Little is known about the life cycle and method of transmission of this nematode; however oral ingestion or wound contamination followed by hematogenous distribution is suggested.7
Based on the granulomatous nature and the lack of hemorrhages, the verminous encephalitis encountered in our case reveals a chronic stage. The presence of nematodes with rhabditiform esophagus, in this case, suggests infection with the nematode Halicephalobus gingivalis. Attempts to definitively identify the organism with PCR were unsuccessful, and this could possibly be attributed to prolonged formalin fixation and processing procedures. Nevertheless, this case represents one of the rare equine verminous encephalitides reported in Australia.
JPC Diagnosis:
Conference Comment:
Conference participants identified numerous tangential cross sections of larval and adult rhabditid nematodes ranging in size from 10-25 um in diameter within areas of granulomatous and necrotizing inflammation randomly scattered throughout the cerebellum. H. gingivalis has a thin smooth cuticle, platymyarian-meromyarian musculature, a pseudocoelom, and a highly characteristic rhabditiform esophagus composed of a corpus, isthmus and bulb.2,3,6,16, Additionally, the intestinal tract is lined by uninucleate, low cuboidal cells and a single genital tract. Participants also recognized occasional 15-20 um ovoid embryonated eggs adjacent to adults and larvae.3,6,16 Interestingly, only female adults, larvae, and eggs are identified within tissue sections. Free-living adult males have been found in the environment, but there are no reports of male H. gingivalis within host tissue. This likely indicates that H. gingivalis adults sexually reproduce in the environment and asexually within hosts.2,3,6,16 The ability of the female parasite to reproduce parthenogenically within the host likely explains the massive numbers present within most affected tissue. Despite its small size, H. gingivalis can produce extensive tissue damage secondary to aggressive migratory behavior, typical of rhabditoid parasites.2,6,16
H. gingivalis usually causes severe perivascular granulomatous and eosinophilic inflammation. Organs other than the brain commonly infected by this parasite include the kidneys, lymph nodes, spinal cord, and adrenal glands. Additionally, tissue within the oral and nasal cavity also commonly contain granulomatous inflammatory lesions.2,3,6,16 Lesions within the heart, liver, stomach, and bones are less common in horses. As mentioned by the contributor, little is known about the pathogenesis and life cycle of this parasite. Oral ingestion, inhalation or wound contaminations have all been proposed as portals of entry. There is also a single case report of probable transmission from a dam to a foal through the colostrum.2,3,16 After entry, the nematode likely disseminates hematogenously throughout the body causing lesions in several organs. In this case, no additional lesions other than the brain are reported by the contributor. In horses, there are case reports with localized infection of the brain, without any other parasite associated lesions elsewhere in the body. It is thought that in those cases, the parasite is inhaled into the nasal cavity and enters the brain via penetration through the cribriform plate.2,3,16
References: