WSC 2023-2024, Conference 19, Case 1
Signalment:
Adult female corn snake (Pantherophis guttatus)
History:
An adult corn snake housed as part of a teaching colony presented for a two week history of regurgitation. During physical examination, a coelomic mass-effect was palpated as well as scale changes that prompted a scrape. Mites were identified and confirmed as Ophyionissus natricis. Supportive care was initiated. The snake was found dead in its enclosure several days after initial examination.
Gross Pathology:
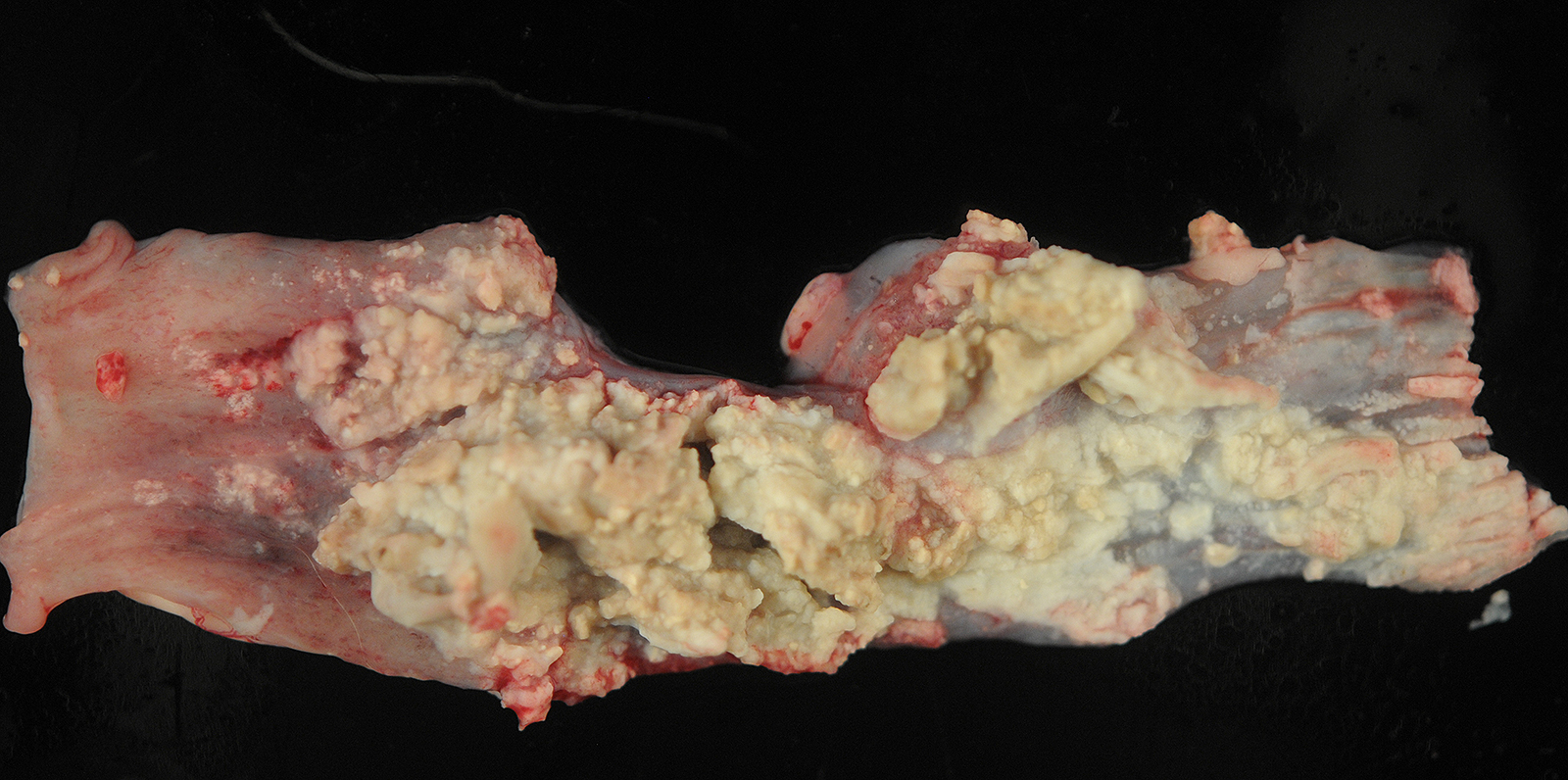
The pleural surface is covered in a thick mat of fibrin. On cut section, there are multifocal 0.1-1.0 cm diameter abscesses containing caseonecrotic exudate.
Laboratory Results:
PCR on fresh gastrointestinal tissue was positive for Salmonella sp. and subsequent culture/genotyping identified S. enterica ssp. enterica serogroup C.
PCR on formalin-fixed paraffin embedded gastrointestinal tissue was positive for Entamoeba invadens.
Microscopic Description:
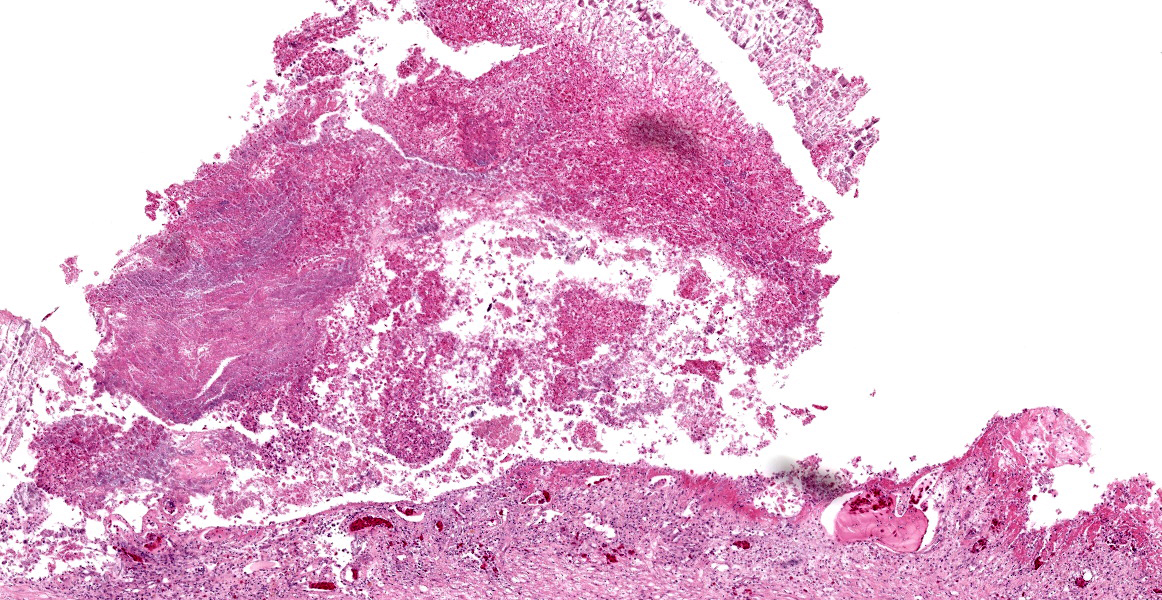
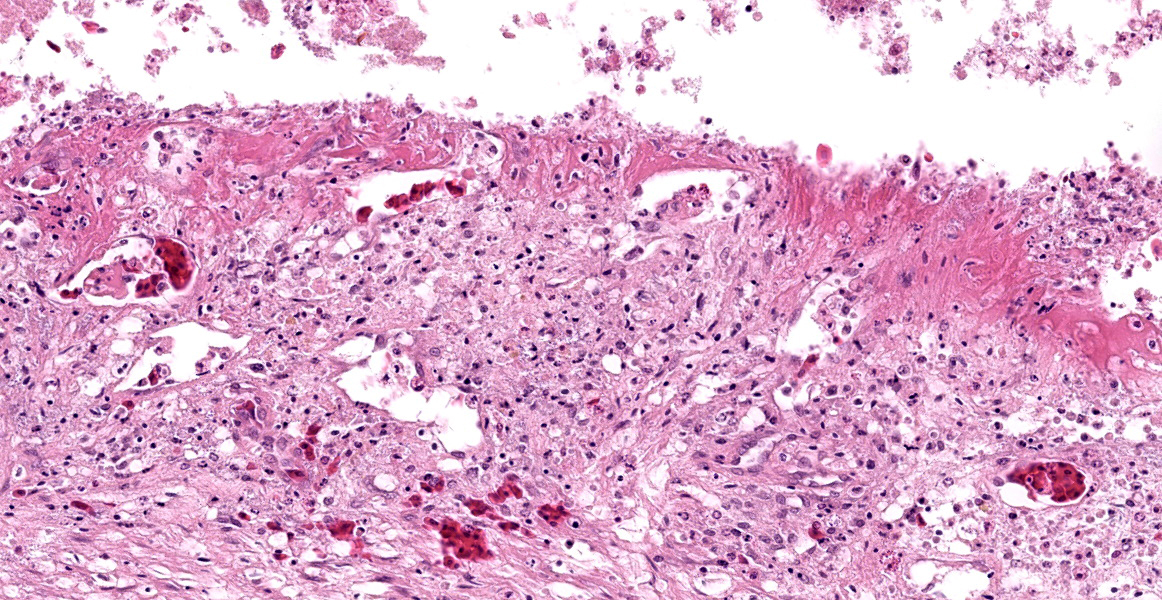
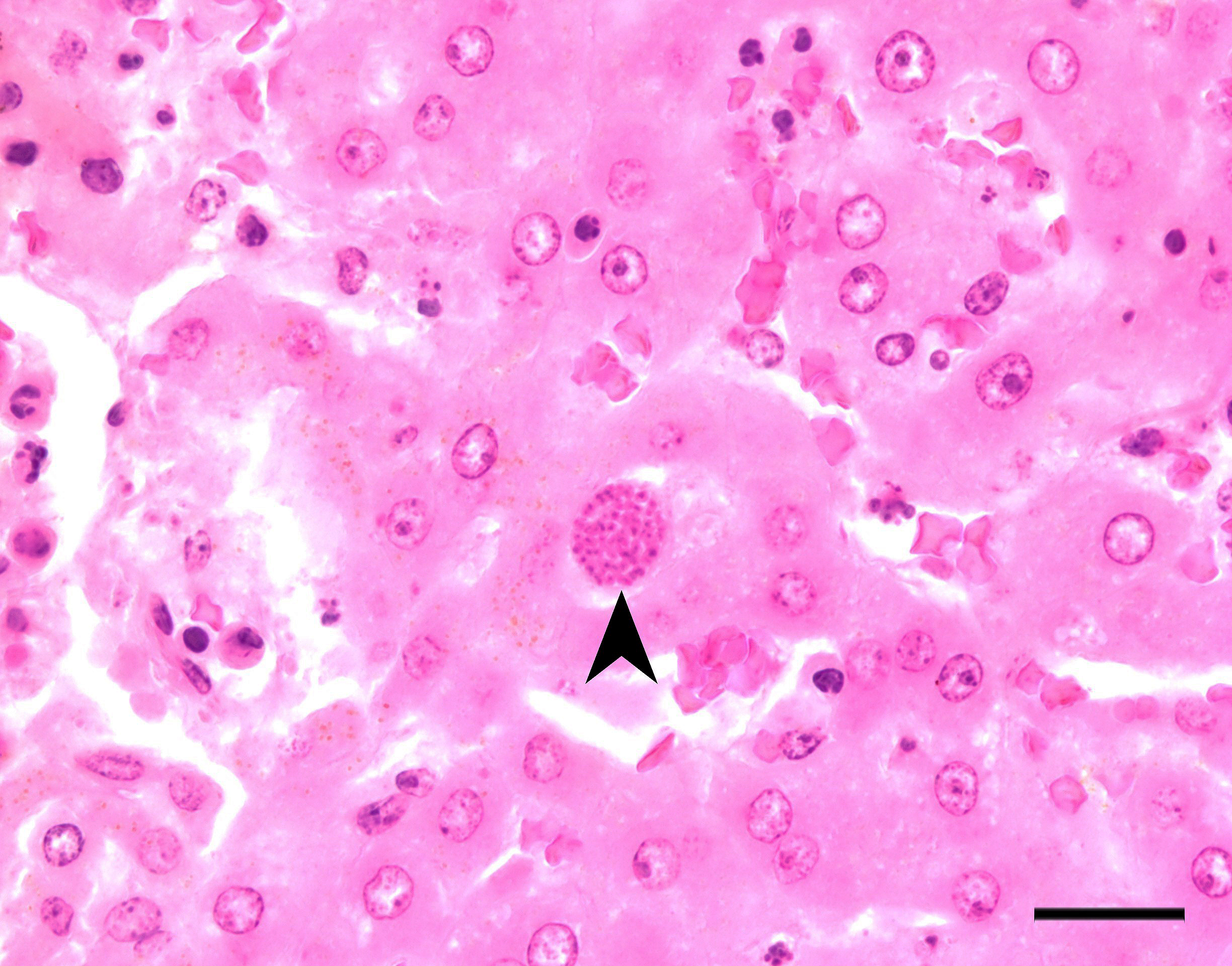
Stomach, ileum and colon: Multifocally the mucosa is eroded to ulcerated and overlain by thick mats of abundant fibrin mixed with cellular and karyorrhectic debris, numerous bacterial colonies, and marked histiocytic and heterophilic inflammation (diphtheritic membrane). In areas of ulceration where the membrane has been lifted, the exposed submucosa is often lined by numerous round protozoal organisms measuring 7-10 µm in diameter with a thin basophilic wall filled with fibrillar to granular basophilic cytoplasm and a single, often eccentrically placed, round basophilic nucleus 2-3 µm in diameter (ameboid trophozoites). Affected submucosa is sometimes expanded by granulation tissue and fibrin thrombi are frequently present within submucosal capillaries. Rarely, necrosis and inflammation extend into the tunica muscularis but does not breach the serosal membrane.
Special stains: Periodic acid-Schiff (PAS) stain applied to sections of gastrointestinal tract highlights the cell wall of amoebic trophozoites.
Contributor’s Morphologic Diagnosis:
Gastrointestinal tract: Severe multifocal, segmentalfibrinonecrotic and histiocytic enteritis with intralesional amoebic trophozoites, diphtheritic membranes and fibrin thrombi.
Contributor’s Comment:
Of amoebic infections in reptiles, Entamoeba invadens is one of the more significant pathogenic species.4,7 Turtles and crocodiles are considered host reservoirs for the organism and transmission follows the fecal-oral route.4,7 Infective cysts are shed in feces and can persist in the environment for prolonged periods.4 Ingestion of cysts by susceptible species results in enteritis, as seen in this case, with some cases extending to the liver as well.4,7 Rare systemic infections have been reported.4 The pathogenesis of Entamoeba invadens is similar to Entamoeba histolytica, a pathogenic amoeba that causes dysentery in humans and nonhuman primates.9,14 E. invadens is often used as a laboratory model for E. histolytica due to its ability to undergo encystation in vitro, which E. histiolytica lacks.7,9
The life stages of Entamoeba species include the transmissible cyst and the infective trophozoite.10 Hosts ingest cysts from contaminated environments and the cyst hatches into a trophozoite via “excystation” within the intestinal lumen.10 Trophozoites then attach to epithelial cells inducing cell injury and death through a variety of mechanisms.2 Attachment of trophozoites to mucins coating the intestinal mucosa and enterocytes occurs via the amebic Gal/GalNAc lectin.2,10 Trophozoites then induce cell death by releasing pore-forming polypeptides called amoebapores and by biting off portions of viable cells, a phenomenon referred to as trogocytosis.2 Both result in increased intracellular calcium, culminating in cell death.
Amoeba-induced enterocyte death results in ulcerative lesions of the gastrointestinal tract and exposes the underlying stroma into which trophozoites can invade by releasing proteases that break down the extracellular matrix.2 This invasion allows for passage of trophozoites into the blood vessels where they can colonize extraintestinal sites in an embolic fashion. In particular, the liver is thought to be infected through a hematogenous route from the portal vein, resulting in liver abscesses.12 Some trophozoites transform back into cysts via “encystation” which are passed into the feces to perpetuate the life cycle.10
Severity of disease may be affected by the environment (namely temperature), host immune status, and protozoal virulence factors.4,7 Additional host factors may include diet. Typically, carnivorous lizards and snakes develop disease with E. invadens infection while herbivorous turtles infrequently develop lesions.7 This may be due in part to the protective gut microenvironment that an herbivorous diet imparts. Virulence of E. invadens, namely encystation and invasion into tissues, decreases in environments rich in glucose and gram-negative bacteria.7,9,14 When comparing the diets of studied reptiles and chelonids, the majority of animals on a carnivorous diet developed E. invadens-induced lesions while those on omnivorous and herbivorous diets had intermediate and low rates of lesion development.7
Aside from E. invadens, snakes can develop disease from E. ranarum infection.8,11 Both can present as a necrotizing gastroenteritis, though extra-intestinal lesions have not been reported with E. ranarum.4,8,11 For cases of E. invadens that only manifest as intestinal disease, as was seen in this case, ancillary testing is needed to definitively determine the species present. Moreover, it is difficult to distinguish between amoebic species on routine histologic examination and so molecular testing, namely polymerase chain reaction assays, have been developed to distinguish between species.1,4 Special stains can be performed to highlight certain features of the organisms. PAS will stain the walls of cysts and trophozoites while trichrome stain can identify certain cellular features such as the dark pink chromatoid bodies.4 The latter is best visualized in fecal preparations.4
Differential diagnoses for gastroenterocolitis in snakes include salmonellosis and coccidiosis.4 While Salmonella spp. PCR and cultures were positive in this case, the yielded bacterium, Salmonella enterica ssp. Enterica, is considered a commensal in reptiles.4 It’s possible dysbiosis secondary to intestinal amebiasis led to overgrowth of S. enterica ssp. enterica and contributed to morbidity in this case. Coccidiosis was also considered based on routine histologic evaluation due to the relatively smaller size of the organisms than is previously reported in cases of E. invadens;4 however, fecal analysis of cohabitants of this snake did not find coccidian organisms and PCR detected E. invadens in this case.
Contributing Institution:
Department of Population Healthan d Pathobiology
North Carolina State University
College of Veterinary Medicine
https://php.cvm.ncsu.edu/
JPC Diagnoses:
1. Gastrointestinal tract: Gastroenterocolitis, necrotizing, multifocal, moderate, with numerous flagellates and superficial bacteria.
2. Kidney: Urate stasis with rare gouty tophi.
3. Presumed ovary: Granulomas, multiple.
JPC Comment:
The contributor provides an excellent overview of enteric disease caused by Entamoeba invadens and the virulence factors that make Entamoeba spp. in general so damaging. As the contributor notes, the organisms in this case are smaller (approximately 4 µm in diameter) than reported for E. invadens, which drew additional, careful scrutiny during conference discussion.
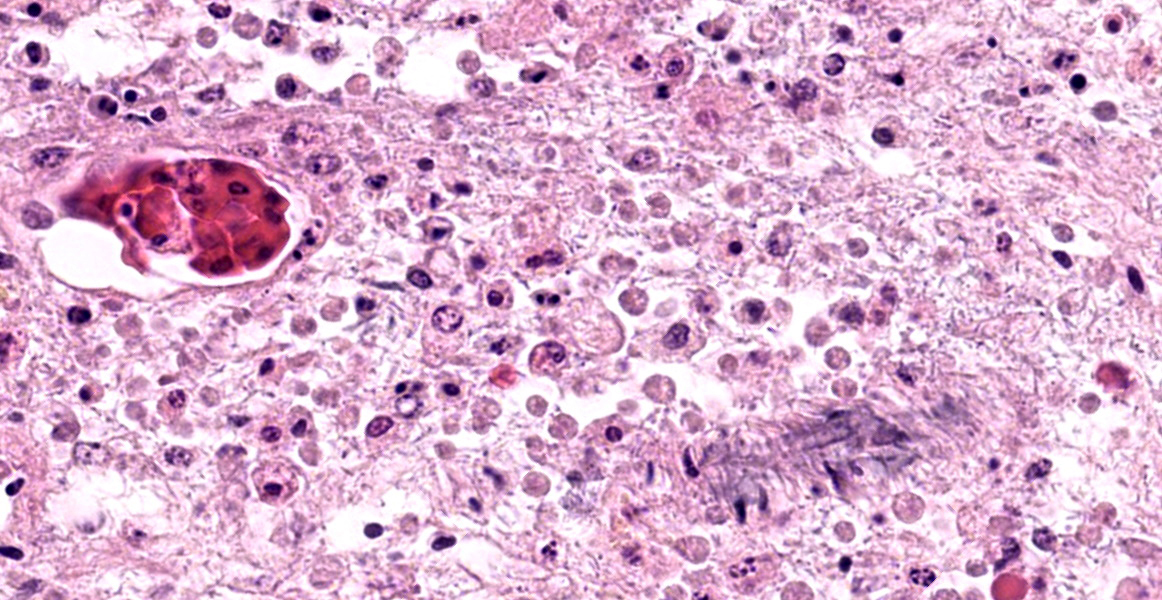
In additional to their small size, the examined protozoa are also irregularly shaped and have eccentric, small, darkly basophilic, nuclei measuring approximately 1 µm in diameter. The cytoplasm is occasionally tapered at one end into a thin filament, which is interpreted as a flagellum. The protozoa are often present in large clusters of dozens of organisms and are overlying ulcerated epithelium.
These characteristics are most consistent with infection with flagellates, which commonly infect the intestines of snakes and are associated with ulceration and secondary bacterial infections.13 Flagellates infect similar anatomic regions as Entamoeba sp., which can make differentiation of these protozoa challenging. Entamoeba invadens are larger (10-15 µm in diameter) than flagellates and have discrete round nuclei Entamoeba invadens typically occur individually (rather than in clusters of dozens of organisms) and are more invasive with more necrosis, hemorrhage, and inflammation than the flagellates seen in this case.13
Interestingly, a previous publication investigated 51 snakes with histologically suspected Entamoeba invadens infection. The authors used immunohistochemistry to confirm or rule out Entamoeba infection. Only 22 cases were confirmed as Entamoeba invadens; all other cases were intestinal flagellate infections with histological feature similar to the present case. Histologic lesions secondary to flagellate infection included inflammation and ulceration of the intestines with a fibrinonecrotizing membrane, and flagellates were reported on the surface of ulcerated mucosa, as seen in this case.5 This work underscores the histologic similarity between Entamoeba invadens and flagellate infections in snakes, and the present
case serves as an excellent example of the difficulty in differentiating these infections.
Flagellates that cause enteritis in snakes are not well characterized. Most cases are assumed to represent Monocercomonas spp. based on the few reports of this flagellate in snake enteritis lesions; however, confirmation of the genus of infective flagellates is rarely performed in cases of snake enteritis.15 Flagellates can infect other organs in reptiles. Recent reptile work has described renal flagellates, suspected of finding their way to the kidney via reflux into the urinary system from the cloaca; flagellate-associated gastroenteritis was also commonly reported in that case series.6 Conference participants did find rare amoebas of the appropriate size within the luminal contents, consistent with the contributor’s PCR results; however, snakes can be asymptomatic but PCR positive for Entamoeba invadens, which is suspected in this case.3
This week’s conference was moderated by Dr. David Needle, Associate Professor at the University of New Hampshire and Senior Veterinary Pathologist and Pathology Section Chief at the New Hampshire Veterinary Diagnostic Laboratory. Dr. Needle led participant discussion of this description-rich slide, which, after much discussion of the etiologic agent as previously detailed, moved to more ancillary lesions, including the gouty tophus within the kidney. Participants noted the abundant pigment within the renal tubules, which is a normal finding in reptiles and is of unknown origin. Participants also noted the unidentified tissue surrounded by macrophages with intracytoplasmic yellow pigment, which participants thought might represent degenerative changes within aged ovaries, though mycobacterial granulomas could not be ruled out in the absence of acid-fast staining.
Consideration of the morphologic diagnosis recapitulated much of the previous conference discussion, with participants convinced that the gastrointerstinal pathology was caused by the flagellates aggregated within areas of tissue necrosis rather than by the occasional amoeba found within the lumen of the gastrointestinal tract.
References:
- Bradford CM, Denver M, Cranfield MR. Development of a polymerase chain reaction test for Entamoeba invadens. J Zoo Wildl Med. 2008;39(2):201-207.
- Espinosa-Cantellano M, Martínez-Palomo A. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev. 2000;13(2):318-31.
- Heng Y, Hsu CD, Mathew A, Oh PY, Li WT, Xie S. Management of Entamoeba invadens in the herpetological collection at the Singapore zoo. J Wildl Med. 2023 Jul;54(2):272-281.
- Jacobson ER, Garner MM. Infectious Diseases and Pathology of Reptiles: Color Atlas and Text. 2nd ed. CRC/Press; 2021:860-862, 904-905.
- Jakob W, Wesemeier HH. Intestinal inflammation associated with flagellates in snakes. J Comp Pathol. 1995;112 (4):417-21.
- Juan-Sallés C, Garner MM, Nordhausen RW, Valls X, Gallego M, Soto S. Renal flagellate infections in reptiles: 29 cases. J Zoo Wildl Med. 2014 Mar;45(1):100-9.
- McFarland A, Conley KJ, Seimon TA, Sykes JM. A retrospective analysis of amoebiasis in reptiles in a zoological institution. J Zoo Wildl Med. 2021;52(1): 232-240.
- Michaely LM, von Dornberg K, Molnar V. Entamoeba ranarum infection in a ball python (Python regius). J Comp Path. 2020;179:74-78.
- Mi-Ichi F, Yoshida H, Hamano S. Entamoeba encystation: new targets to prevent the transmission of amebiasis. PLoS Pathog. 2016;12(10):e1005845.
- Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata, Bhattacharya A, Petri Jr.WA. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature. 2014;508(7497): 526-530.
- Richter B., Kübber-Heiss A., Weissenböck H. Diphtheroid colitis in a Boa constrictor infected with amphibian Entamoeba sp. Vet Parasitol. 2008;153(1-2):164-167.
- Usuda D, Tsuge S, Sakurai R, et al. Amebic liver abscess by Entamoeba histolytica. World J Clin Cases. 2022;10(36): 13157-13166.
- Walden HDS, Greiner EC, Jacobson ER. Parasites and parasitic diseases of reptiles. In: Jacobson ER, Garner MM, eds. Infectious Diseases and Pathology of Reptiles. 2nd ed. CRC Press;2020.
- Watanabe K, Petri WA. Learning from the research on amebiasis and gut microbiome: is stimulation by gut flora essential for effective neutrophil mediated protection from external pathogens? Gut Microbes. 2019;10(1):100-104.
- Zwart, Peernel and SFM, Teunis. Monocercomoniasis in reptiles. September 1982. Conference: First International colloquiem on pathology of reptiles and amphibians. At: Angers (France). 73-76.