Signalment:
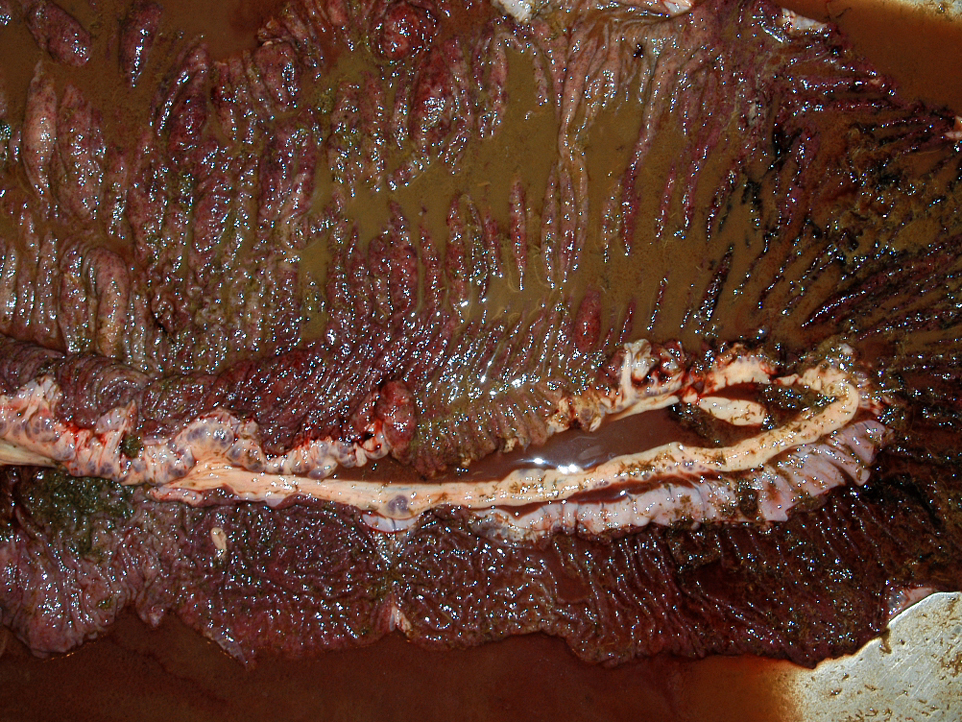
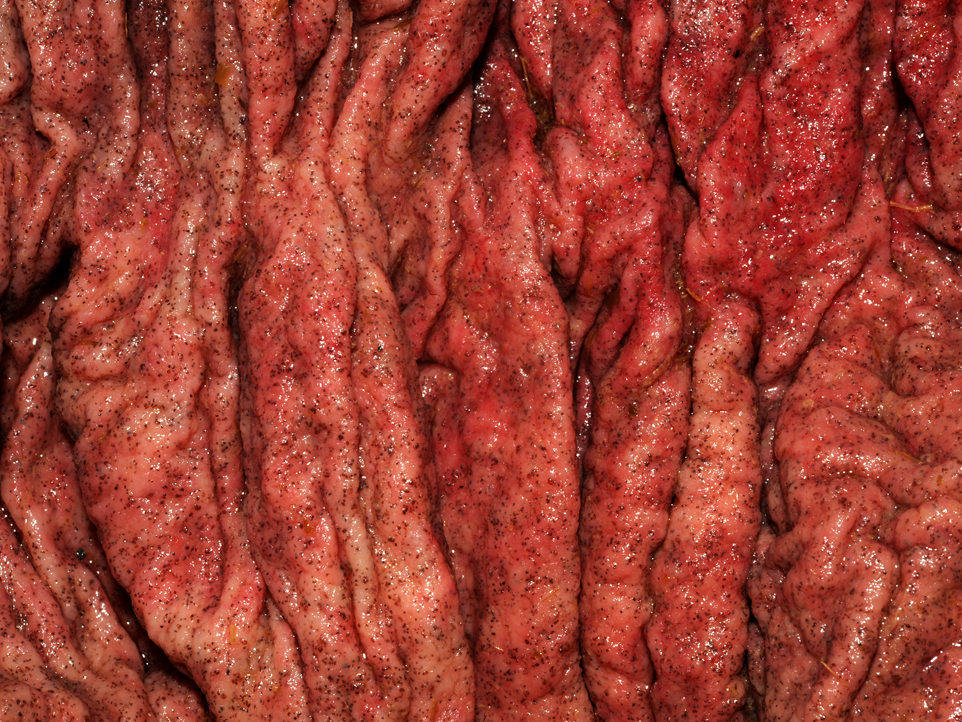
Gross Description:
Histopathologic Description:
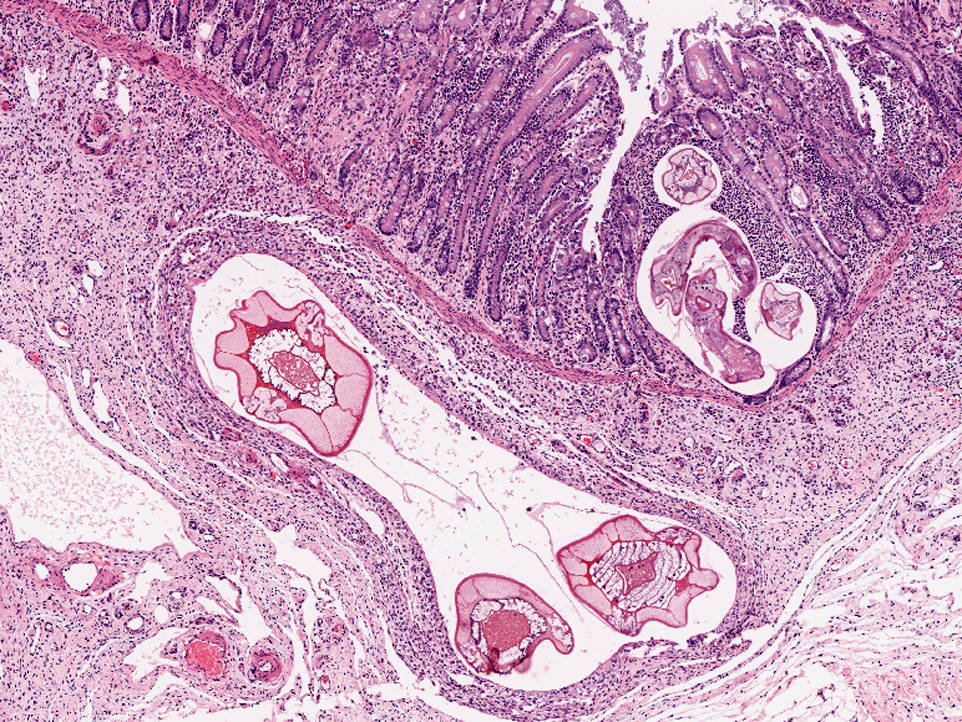
Colon, mucosa: There is focal erosion and ulceration of villus tips; enterocytes exhibit a mild goblet cell hyperplasia. Multifocally enterocytes contain 20-60 μm large, basophilic, PAS negative fine granular intracytoplasmic structures (goblet cell hyperplasia/apicomplexan protozoa?). The lamina propria contains moderate to large numbers of metazoan parasites (nematode larval stages) consistent with small strongylid larvae.Â
Smallest larvae are 20 μm in diameter, up to 100 μm long and crescent shaped with pointed tail tips (L3) with mild peripheral histiocytic infiltration. Fewer L3 stages exhibit very mild or no inflammatory reaction (hypobiotic early third larval stages, EL3).Â
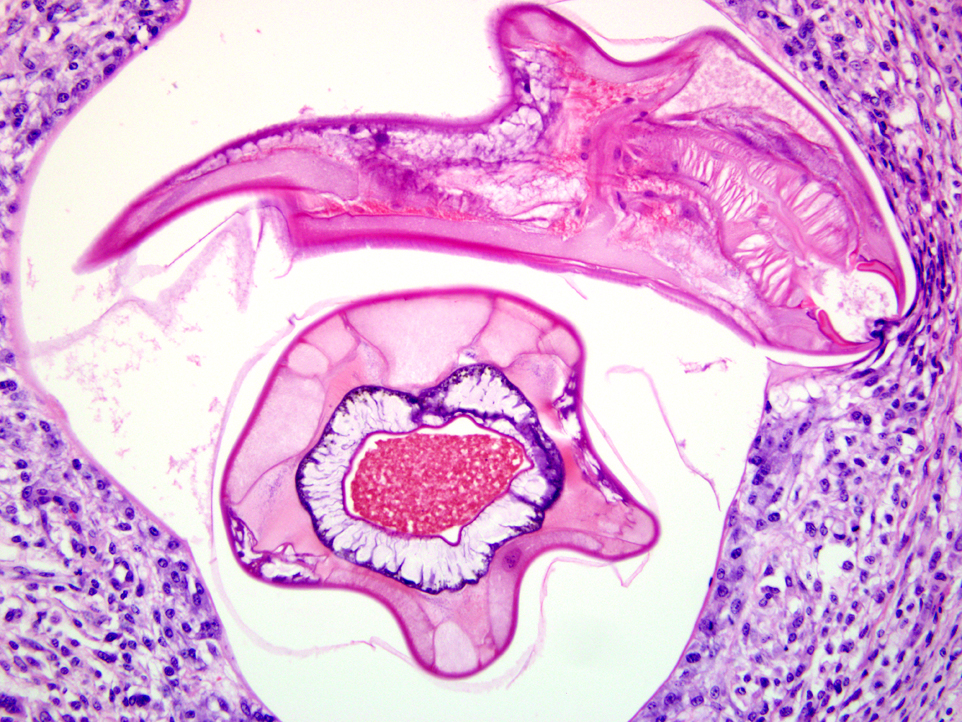
A population of further matured up to 100 μm diameter nematode larvae with an eosinophilic, thick, smooth cuticle, vacuolated lateral cords, prominent platymyarian musculature and intestinal as well as genital tract is observed throughout the lamina propria mucosae (L4). The intestine often exhibits prominent brush borders and these stages multifocally contain large amounts of dark red to dark brown pigment (iron pigment). The inflammatory response ranges from an acute, densely cellular neutrophilic and partly eosinophilic cuffing (emerging larvae?), to a capsule-like formation composed of macrophages and fibroblasts.Â
Few migrating L4 stage larvae (not present in all sections) associated with superficial epithelial erosion and ulceration, acute haemorrhage and mild to moderate neutrophilic and eosinophilic infiltration is observed.Â
A mild to moderate diffuse infiltration by lymphocytes, plasma cells, lesser histiocytes and neutrophils and focal acute hemorrhages and few crypts containing cellular debris as well as neutrophils (crypt abscesses) is present in the mucosa.Â
Submucosa: Parasitic nematodes within the submucosa are much larger, 200 μm in diameter, with nematode specific external and nematode intestinal structures often containing erythrocytes, indicating blood uptake. A moderately dense histiocytic infiltration admixed with proliferating fibroblasts is surrounding the majority of these stages. The submucosa is generally expanded by clear spaces (moderate edema) as well as a multifocal to confluent mixed cellular (lymphocytes, plasma cells, macrophages, lesser neutrophils and occasional eosinophils) infiltrate.
Morphologic Diagnosis:
Lab Results:
Clinical pathology:
| PCV | 48% |
| TP | 56g/l |
| Albumin | 21g/l |
| Globulin | 35g/l |
| WBC | 14.10 x 109 |
| Lymphocytes | 11% |
| Neutrophils | 86% |
| Na | 111 (126-146) mmol/l |
| K | 1.2 (3.0-5.0) mmol/l |
Parasitology: Colonic content was subjected to a parasitological worm egg screen examination. No eggs were detected.
Condition:
Contributor Comment:
More than 50 nematode species from subfamilies of Cyathostominae (~44 species), Strongylinae (minimum 11 species), and Gyalocephalinae, popularly known as trichonemes, cyathostomes or cyathostomins are embraced by the group -�-�small strongyles.(10) Small strongyles range from 0.4 to 1.5 cm length in male animals and 0.5 to 2.0 cm length in female animals and are overall smaller than large strongyles, although some species may reach 2 cm to 3 cm in length.(9) Adults of some species inhabit the cecum or dorsal and ventral colon, with others inhabiting just one of these intestinal compartments. Adult small strongyles feed on enterocytes / enterocytic cellular debris or penetrate the epithelial barrier, open small vessels and digest blood.Â
In consistency with large strongyles, small strongyles have a direct life cycle with no intermediate hosts. Eggs are passed through the feces onto pasture. Depending on environmental conditions, eggs may hatch and develop from the first stage larvae (L1) to infectious L3 stages in as few as three days. Once ingested by the equine host, they continue to mature, and, in a rapid life cycle, new eggs may be passed onto the pasture in as few as 5-6 weeks. Larval development of most species takes place entirely in the mucosa of the cecum and colon, but few penetrate the muscularis mucosae and develop within the submucosa.(3)
In the present case, large nematode larvae containing ingested erythrocytes were observed in the submucosa. Due to their size and ingestion of blood, larval migration of large strongyle species has to be considered. It has been shown that L3 of large strongyle species exsheath in the small intestine and penetrate the mucosa and submucosa of the small intestine, caecum and colon within 13 days where they moult to the fourth stage larvae (L4) by about day 7.(4) Larval stages of different small and/or large strongyle species are not distinguishable histologically and therefore, co-infection cannot be ruled out.
The entry of small strongyle larvae into tubular gland lumina generally provokes a chronic inflammatory response and marked goblet cell hyperplasia. Emergence of L4 can be associated with an acute eosinophilic infiltration. Cyathostomins differ from other worm species in that the maturation of early third stage larvae (EL3) can be arrested for prolonged periods of time (hypobiosis). After ingestion, L3 exsheath and invade the large intestinal mucosa. Here, larvae protect themselves by becoming encysted and the host generally only reacts with a minimal inflammatory response to those stages. Up to 90% of an equine worm burden may become hypobiotic and EL3 stages can remain within the intestinal walls for 4 months up to as long as 2 years.(3)
The majority of clinical signs associated with heavy infections in horses up to 2-3 years of age are unthriftiness, anemia and sometimes diarrhea. A typical clinical picture can include neutrophilia, hypoalbuminemia, hyperglobulinemia, low total serum protein as well as slightly high total protein, possibly due to dehydration, findings most consistent with protein-losing enteropathy.(7) Marked clinical signs are less common in older animals, though protective immunity never develops. However, the most significant damage can arise from simultaneous mass emergence of encysted L4 larvae (-�-�larval cyathosominosis) continuing their development to the adult stage in the intestinal lumen. Catarrhal and/ or hemorrhagic enteritis with severe diarrhea, potentially serious colic, leading to emaciation and in some cases death (mortality rate up to 50%) can be caused by severe damage to the gut wall. The climate is responsible for the occurrence of hypobiotic stages. In temperate climates larvae will accumulate during grazing season, encyst during the cooler months and re-emergence may occur en-masse as spring sets in. Reversed timing is observed in tropical climates where larval hypobiosis generally occurs during hot, stressful summer months with larval emergence in autumn.(1,7)
The antemortem diagnosis of larval cyathostominosis can, due to presentation of nonspecific clinical signs, be challenging. Depending on clinical findings, a variety of gastrointestinal diseases (e.g. salmonellosis, nonsteroidal anti-inflammatory drug-induced colitis, intestinal lymphosarcoma, inflammatory bowel disease, infections with Lawsonia intracellular is or Clostridium spp.) as well as other causes of ill-thrift and hypoproteinemia in nondiarrheic animals (e.g. renal disease, peritonitis, malnutrition) have to be considered as differential diagnoses.(8) Diagnosis in the live horse can be attempted by the fecal flotation method. However, this method can be unreliable because of variation in larval survival rates under certain culture conditions, is time-consuming and labor intensive and can be falsely negative or low because of the absence or paucity of adult nematodes. Therefore, definitive antemortem diagnosis is often not possible.Â
However, a presumptive diagnosis can be made based on signalment, clinical signs and exclusion of other possible causes. Post mortem diagnosis generally reveals strongly indicative findings. Additionally, transillumination is an easily employable method to detect intramucosal larvae and provides a fast and definitive diagnosis. Recently, investigations into internal transcribed and intergenic spacers of nuclear rDNA have been undertaken and these markers have proven to be useful for the identification of Strongylinae and Cyathostominae species. Importantly, the PCR-based methods developed using these markers can be sensitive and specific for diagnosis, and therefore might, via the detection of parasitic DNA extracted from intestinal biopsies, provide a useful diagnostic tool for intra vitam diagnosis of larval cyathostominosis.(4,6)
JPC Diagnosis:
Conference Comment:
The moderator discussed the importance of careful preparation and examination of the luminal surface of the affected intestine, because the characteristic gross appearance of cyathostomiasis with multiple red to black pinpoint nodules diffusely covering the mucosa can easily be missed if the digesta or feces is not carefully washed from the mucosal surface. Also, the mucosa and submucosa become very edematous and congested but not hemorrhagic, and will easily tear if palpated carelessly.Â
The process of massive emergence of hypobiotic larvae causing disease in otherwise clinically normal horses is similar to the phenomenon in cattle with type II disease as a result of Ostertagia spp. Ostertagiosis is the most important parasitic disease in grazing cattle and sheep in temperate climates, resulting in production loss and death. Lesions include severe abomasal mucous metaplasia, epithelial hyperplasia, and interstitial inflammation. This results in abomasal alkalosis, edema, and significant protein loss.(2)
References:
2. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Edinburgh, Scotland: Saunders Elsevier; 2007:233-4, 248-9.
3. Corning S. Equine cyathostomins: a review of biology, clinical significance and therapy. Parasit Vectors. 2009;2 (Suppl 2): S1.
4. Gasser RB, Hung GC, Chilton NB, et al. Advances in developing molecular-diagnostic tools for strongyloid nematodes of equids: fundamental and applied implications. Mol Cell Probes. 2004;18:3-16.
5. Lyons ET, Tolliver SC, Drudge JH. Historical perspective of cyathostomes: prevalence, treatment and control programs. Vet Parasitol. 1999;85: 97-111; discussion 111-112, 215-125.
6. Matthews JB, Johnson DR, Lazari O, et al. Identification of a LIM domain-containing gene in the Cyathostominae. Vet Parasitol. 2008;154:82-93.
7. McWilliam HEG, Nisbet AJ, Dowdall SMJ, et al. Identification and characterisation of an immunodiagnostic marker for cyathostomin developing stage larvae. International Journal for Parasitology. 2009;40:265-275.
8. Peregrine AS, McEwen B, Bienzle D, et al. Larval cyathostominosis in horses in Ontario: an emerging disease? Can Vet J. 2006;47:80-82.
9. Rommel M, Eckert J, Kutzer E, et al. Parasitosen der Einhufer. In: Buchverlag P, ed. Veterinaermedizinische Parasitologie. Berlin, Germany: Blackwell; 2000:367-395.Â
10. Taylor MA, Coop RL, Wall RL. Parasites of horses. In: Veterinary Pathology. 3rd ed. Hong-Kong: Wiley-Blackwell; 2007:272-273.Â