Signalment:
7-year-old castrated, male paint horse (
Equus caballus).Small, non-visual left eye for the past one year; clinically suspected chronic uveitis. The right eye was normal.
Gross Description:
Globe was somewhat small with a cloudy cornea.
Histopathologic Description:
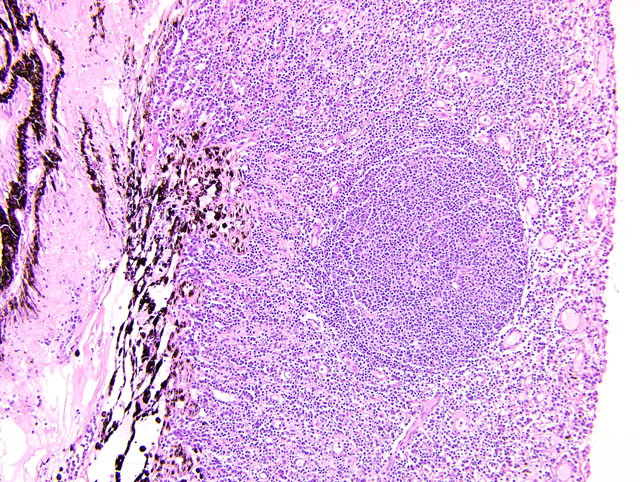
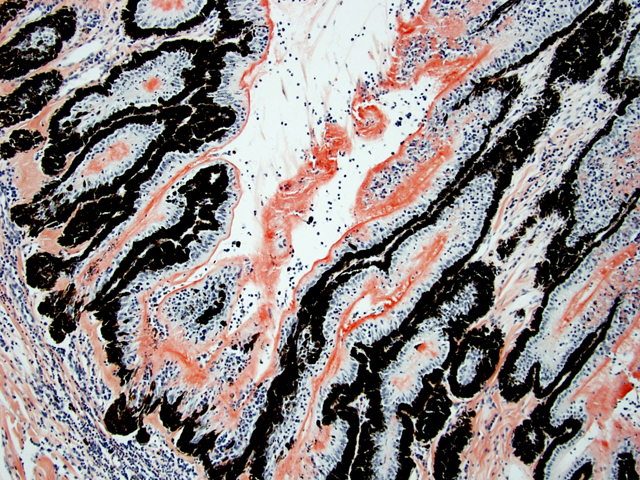
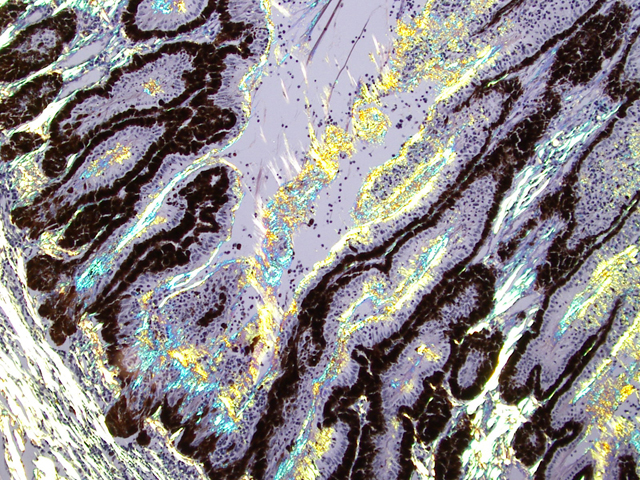
Eyeball (a-d): The cornea contains a modest amount of neovasculature within the superficial third of the stroma. The anterior chamber is filled with eosinophilic flocculent material (serous exudate). The iris is greatly thickened and distorted; it contains a marked perivascular to diffuse infiltrate of plasma cells and lymphocytes. In addition, the anterior and posterior surfaces are covered with a layer of fibrovascular tissue (preiridial fibrovascular membrane) and adherent plasma cells and lymphocytes, which spans the pupil and is adhered to the anterior lens capsule (posterior synechia). The filtration angle is variably collapsed and the ciliary cleft contains a moderate accumulation of lymphocytes, plasma cells, and melanophages. The ciliary body is distorted and contains an infiltrate similar to the iris; in addition, it contains scattered follicular aggregates of lymphocytes and plasma cells. Also, the neuroepithelium of the ciliary body is covered by a thick layer of homogeneous eosinophilic, congophilic material that has a somewhat ragged surface; this material has green birefringence with polarized light. There is a layer of fibrillar fibrous material extending from the surface of the ciliary body along the surface of the posterior lens capsule (cyclitic membrane). The choroid is diffusely congested and contains a few scattered aggregates of lymphocytes and plasma cells. The retina is detached in several places and the subretinal space contains eosinophilic flocculent material and a few melanophages. The retinal pigment epithelium is hypertrophied in the corresponding detached areas. The nontapetal retina is thin due to a thinned nerve fiber layer, thinned inner and outer plexiform layers, a diminished number of ganglion cells, and a hypocellular inner nuclear layer, which somewhat blends into the outer nuclear layer. Ganglion cells that are present have central chromatolysis. The photoreceptors are evident but blunted. There are a few folds in the retina. The tapetal retina is more nearly normal. The lens capsule is ruptured in several foci, especially on the anterior surface adjacent to the posterior synechiae. In these areas, the anterior cortical lens fibers are swollen, fragmented and some have undergone fibrous metaplasia, which has produced a thick fibrous layer that covers the inner surface of the anterior lens capsule. The vitreous body has deteriorated and the vitreous chamber is filled with eosinophilic flocculent material (plasmoid vitreous). The bulbar conjunctiva at the limbus contains a marked follicular infiltrate of lymphocytes and plasma cells.
Morphologic Diagnosis:
Eyeball: Chronic lymphoplasmacytic uveitis (especially anterior uveitis) with posterior synechiae, partial filtration angle closure, cortical cataract with capsule rupture and fibrous metaplasia, retinal detachment and retinal degeneration, non-tapetal retina.
Condition:
Equine recurrent uveitis
Contributor Comment:
This eye has chronic lymphoplasmacytic uveitis (especially anterior uveitis) with many secondary changes that include posterior synechiae, partial filtration angle closure, cortical cataract, retinal detachment and retinal degeneration. The pattern of the uveitis with the accumulation of congophilic material (amyloid) on the ciliary body is typical of equine recurrent uveitis (ERU). ERU is a fairly common form of uveitis in horses; apparently there is breed predisposition, with Appaloosas being very susceptible and, when affected, having bilateral involvement about 80% of the time. Some aspects of the cause and pathogenesis are not clear, but the consensus seems to be that the uveitis develops due to an autoimmune reaction. The prevailing theory is that certain bacterial antigens, most notably those on certain serovars of
Leptospira interrogans (especially
L. pomona) are similar to intrinsic antigens in the uvea, and that the immune response generated against the bacterial antigens cross-reacts with the uveal antigens resulting in autoimmune mediated inflammation.(1) The retinal degeneration has a pattern that is consistent with secondary glaucoma (non-tapetal involvement) but also could have been caused by ocular hypertension, a condition that often accompanies ERU.(1)
JPC Diagnosis:
Eye: Endophthalmitis, lymphoplasmacytic, chronic, diffuse, mild to moderate, with posterior synechia, pre-iridial fibrovascular membrane, post-iridial amyloid-like material, retinal detachment and atrophy, peripheral corneal neovascularization, and cataractous change.
Conference Comment:
Equine recurrent uveitis (ERU) is one of several immune-mediated endophthalmitides of veterinary importance; others include canine uveodermatologic syndrome, canine lymphocytic uveitis, feline idiopathic lymphonodular uveitis, and lens-induced uveitis. Because ERU is a progressive disease, a wide range of potential microscopic findings, from uveitis to endophthalmitis to phthisis bulbi, exists.(2) This case nicely illustrates several of the characteristic features of ERU, including the hallmark lesion, deposition of hyalinized congophilic (i.e. amyloid-like) material in the apical cytoplasm of the posterior iridociliary epithelium.(2) The presence of numerous additional pathologic findings makes for a very descriptively challenging slide. The retinal lesions, as suggested by the contributor, may be the result of secondary glaucoma; however, glaucoma is a rare complication of ERU, and it is thought that the trabecular meshwork is less important than uveoscleral resorption for aqueous drainage in horses, in contrast to the mechanism of aqueous outflow in dogs and cats.(1,2) Some researchers suggest that a retinopathy may be the primary immunological event in equine uveitis and that interphotoreceptor retinoid-binding protein (IRBP) is the major autoantigen in ERU. This autoantigen, along with others, may be unmasked by a variety of intraocular inflammatory events leading to a common final pathway that is characteristic of ERU. Alternatively, as alluded to by the contributor, the mimicry of autoantigens by infectious agents is a popular hypothesis; a 90-kd protein common to several serovars of
Leptospira interrogans shares epitopes with an equine corneal peptide, lending credence to this hypothesis.(1)
The pathogenesis of immune-mediated endophthalmitis is complex and its discussion is enhanced by a rudimentary understanding of a recently-discovered, carefully-regulated system of ocular immunity called anterior chamber-associated immune deviation (ACAID). Briefly, ACAID is a series of responses to intraocular antigen that ultimately results in impaired delayed-type hypersensitivity (DTH) expression and suppressed complement-fixing antibody production without suppressing antigen-specific cytotoxic T cell activity. As a result, innocent bystander intraocular tissues are spared collateral damage from T cell-driven inflammation.(1) More specifically, a number of cytokines, e.g. TGF-_2, TNF-_, vasoactive intestinal peptide, and substance P, alter intraocular antigens, which are then processed by resident antigen presenting cells (APCs), i.e. macrophages and dendritic cells, within the uvea. The APCs travel to the spleen where they promote the development of specific suppressor T-cells, which then return to the uvea and suppress DTH and complement-fixing antibody production, but not cytotoxic T cell activity. When this system is overwhelmed or impaired by uncontrolled introduction of antigen into the globe, perivascular aggregates of lymphocytes develop in the iris, ciliary body, choroid, and retina, and may eventually form vague lymphoid follicles. Amplification results in decreased specificity for the initial inciting antigen among the acquired lymphoid population and a stereotyped response to circulating antigens that enter the eye through a now-damaged blood-eye barrier.(2)
Taken together, the evidence suggests that ERU is multifactorial, as is the predisposition to its development. The contributor mentioned that Appaloosas are predisposed, with 80% of cases being bilateral. In contrast, only 20% of non-Appaloosas with ERU develop bilateral disease, and Appaloosas with light coats and many spots are more likely to develop ERU than are Appaloosas with dark coats and few spots, suggesting that melanin may be protective. In German Warmblood horses, the MHC I haplotype ELA-A9 is strongly associated with ERU, further supporting the hypothesis of a genetic predisposition.(1)
References:
1. Brooks DE, Matthews AG: Equine ophthalmology.
In: Veterinary Ophthalmology, ed. Gellat KN, 4th ed., pp. 1239, 1244-1248. Blackwell Publishing, Ames, IA, 2007
2. Wilcock BP: Eye and ear.
In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 505-509. Elsevier Saunders, Philadelphia, PA, 2007