CASE III:
Signalment:
Juvenile male Western crow (Corvus brachyrhynchos hesperis)
History:
This case is one in a series of juvenile crows that were part of a large mortality event at the San Diego Zoo Safari Park in the first few weeks of August, 2020. Most crows were found deceased on park grounds and submitted for necropsy.
Gross Pathology:
A juvenile male crow was presented for necropsy. The skin and subcutis were tacky and dry (dehydration). There were minimal to no fat stores, and the pectoral muscles were depressed below the keel (poor body condition). The intestine was segmentally dark purple to green and the ceca were bilaterally plump. The spleen was prominent and dark red.
Laboratory Results:
PCR (Frozen intestine and spleen from select cases): Approximately 50% of samples were positive for orthoreovirus using conventional PCR targeting the L2-genome segment encoding the RNA-dependent RNA-polymerase.
Microscopic Description:
The slide contains two sections of small intestine, one section of large intestine, and one or two sections of spleen.
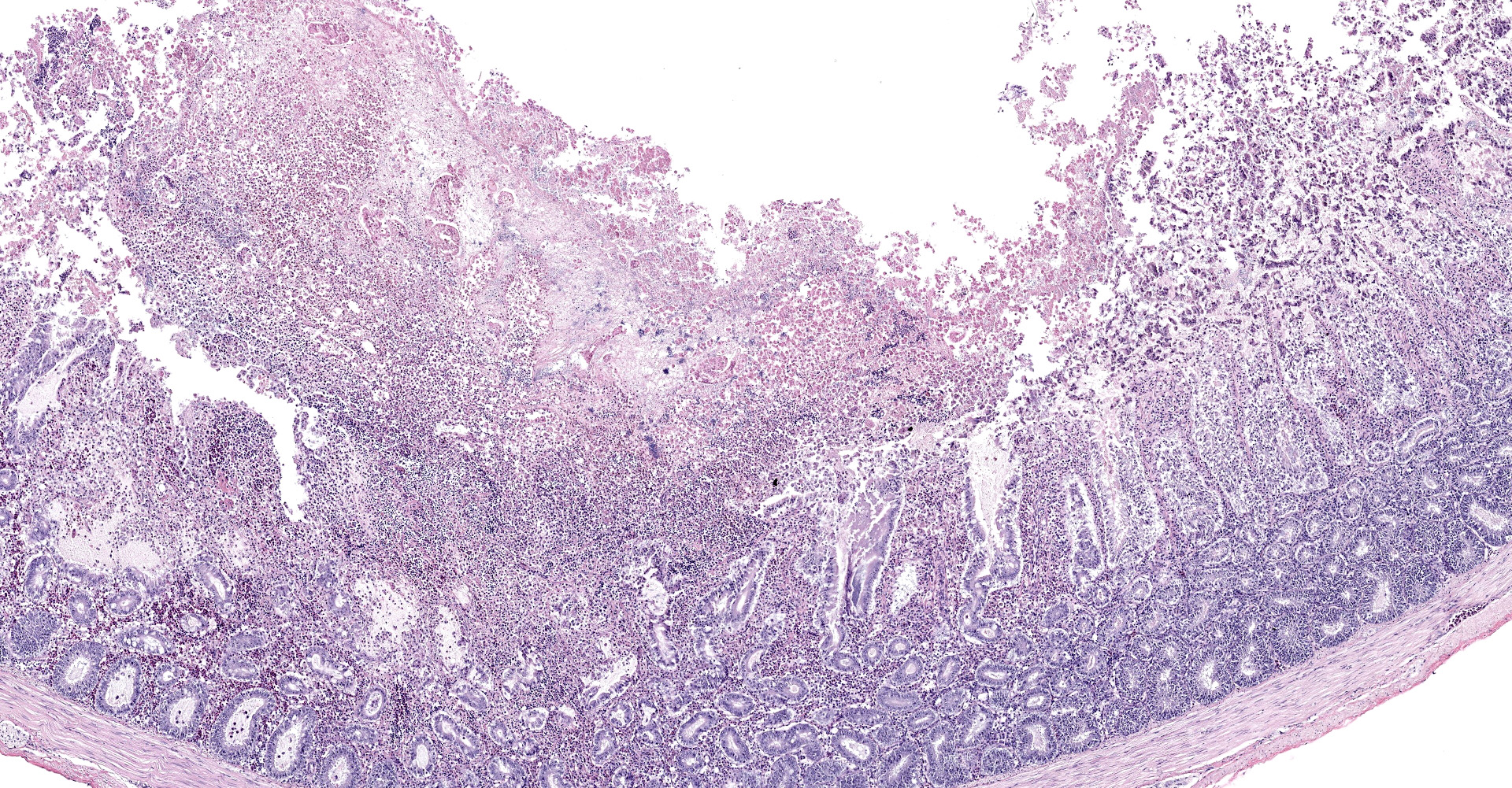
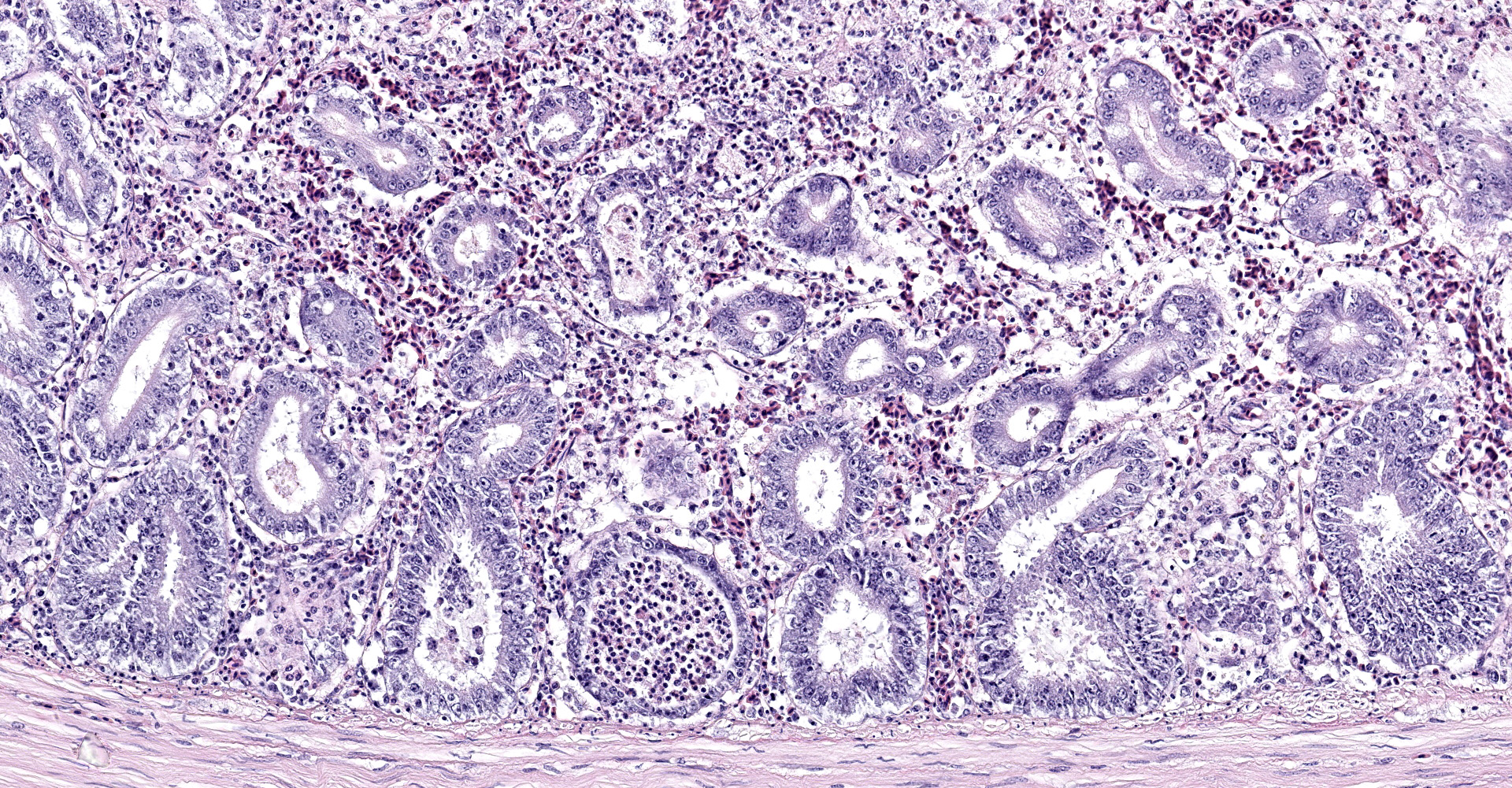
Intestine: All sections of intestine are similarly affected by segmental to circumferential necrosis, characterized by deep ulceration and loss of the mucosa, lamina propria, and submucosa with replacement by abundant, fibrillar eosinophilic material (fibrin), erythrocytes (hemorrhage), and fragmented cellular and nuclear debris admixed with large colonies of bacilli. In less affected segments, necrotic material forms a thick, intermittent pseudomembrane adherent to the remnant mucosal surface, and in more severely affected sections fibrinonecrotic debris fills the entire lumen. Crypts are dilated, distorted, and variably separated, and frequently contain clumps of necrotic cells and pale eosinophilic, flocculent debris. Occasionally, crypts contain small to moderate numbers of luminal and rarely intracellular spirochetes. Crypt epithelium ranges from piled and hypertrophic to flattened and attenuated. The intervening lamina propria is expanded by abundant erythrocytes, heterophils, histiocytes, and aggregates of fibrin, and lymphoid tissues are expanded by clear space and karyorrhectic debris (edema and lymphocytolysis, respectively). Lymphocytes, plasma cells, fewer granulocytes, and erythrocytes dissect within the muscular wall along mural vessels and multifocally extend to the serosa and adjacent mesentery, which is disrupted by aggregates of intact and degenerate leukocytes.
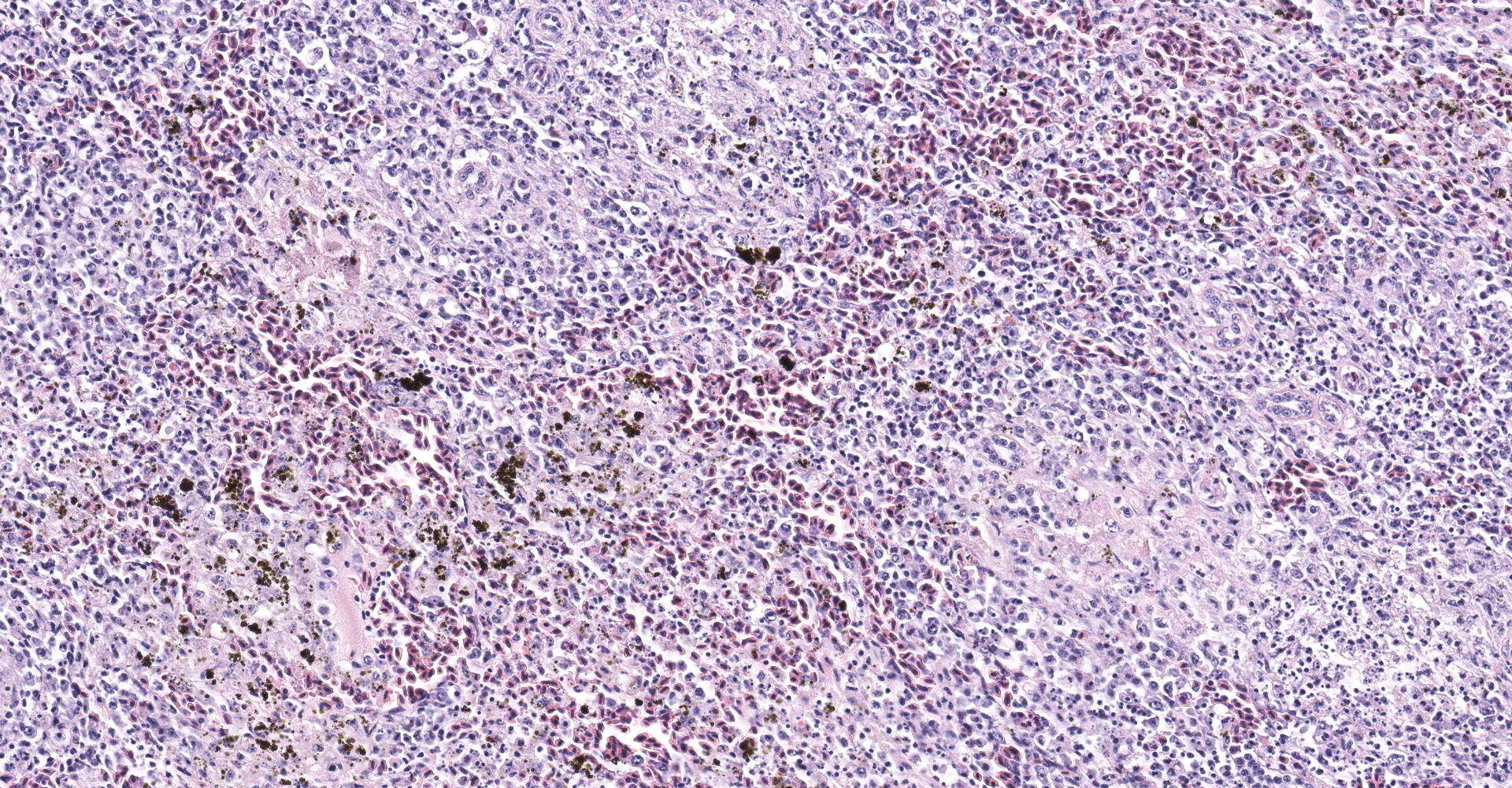
Spleen: The spleen is multifocally disrupted by foci of fibrillar, pale eosinophilic material (fibrin), erythrocytes, and smudged to stippled cellular and karyorrhectic debris (necrosis). Foci are variably associated with aggregates of foamy histiocytes, many of which contain intracytoplasmic, coarsely-granular, golden-brown, birefringent pigment (suspect hemozoin). There are abundant shrunken cells with fragmented and pyknotic nuclei throughout all sections (lymphocytolysis).
Contributor's Morphologic Diagnoses:
1. Small intestine, colon, ceca: Severe, multifocal to segmental, acute, fibrinonecrotizing, hemorrhagic, and heterophilic enterotyphlocolitis
2. Spleen: Moderate multifocal acute fibrinonecrotizing splenitis with mild birefringent pigment deposition (hemozoin, presumptive)
Contributor's Comment:
A large number of crows were submitted to Disease Investigations at the San Diego Zoo over a one month period through August of 2020, all of which were juveniles found dead or moribund. Consistent lesions included severe necrohemorrhagic enterotyphlocolitis, fibrinonecrotizing splenitis, and poor body condition. Additional, less consistent lesions (not submitted) included depletion of lymphocytes in the bursa of Fabricius, myeloid hyperplasia in the bone marrow, and acute renal tubular degeneration and necrosis (possibly dehydration-related). The findings are consistent with those attributed to avian orthoreovirus, which has been reported in American corvids as early as 2001 and was more recently described in a retrospective review of crow mortalities in New York.2 Classically, outbreaks of the virus in crows have occurred during winter months, lending it the name "winter mortality of crows".2 This particular event was unusual in that it occurred in late summer, however it appeared to be associated with large numbers of
gathered crows based on subjective observation, which is a known prerequisite for epizootics.2 Secondary bacterial infections (especially bacterial enteritis) and sepsis were common sequelae in many crows, and some cases had coinfections with hemoparasites, Aspergillus sp., and avian poxvirus.
Avian reoviruses are non-enveloped, double-stranded RNA viruses that are common in commercial poultry, where disease is typically manifested as tenosynovitis.1,4 The virus has been classified into six genotype clusters based on the sigma C protein sequence, which demonstrates the greatest variability between viral isolates.1 Thus far, viral pathogenicity and induced disease has not been linked to a particular genotype.4 There have been several case reports of various corvid species with a novel avian orthoreovirus-associated presentation characterized (predominantly) by necrotizing enteritis and splenitis, including a magpie in Great Britain, a hooded crow in Finland, and American crows in North America.2,5 In the 2019 review by Forzan et al., the authors developed an in situ hybridization probe that was able to demonstrate for the first time direct viral association with the intestinal and splenic lesions in affected crows, strongly supporting causality. Notably, a wide variety of wild birds across multiple orders have also been reported with orthoreovirus-associated disease, including Anseriformes, Pisttacines, and Columbiformes, among others. Documentation of these cases is lacking, however the most consistently identified histopathologic lesion in these species is a necrotizing hepatitis.3
The examined case provides an example of the lesions of avian orthoreovirus in crows and cautions that orthoreovirus should be considered a differential in sudden outbreaks of high mortality in corvids with evidence of enteritis and splenitis, regardless of season.
Contributing Institution:
San Diego Zoo Wildlife Alliance
Disease Investigations
P.O. Box 120551
San Diego, CA 92112-0551
https://science.sandiegozoo.org/disease-investigations
JPC
Diagnosis:
1. Small and large intestine: Enterocolitis, fibrinonecrotizing, hemorrhagic, circumferential, diffuse, severe with crypt and glandular loss.
2. Spleen: Splenitis, necrotizing, multifocal, mild to moderate, with lymphoid depletion.
3. Spleen, macrophages: Hemozoin pigment, moderate.
4. Adipose tissue, mesenteric: Atrophy, diffuse, marked.
JPC Comment:
The contributor provides an excellent introduction to a viral disease of American corvids colloquially known as "winter mortality of crows". This entity was initially detected through the New York State Wildlife Health Program (NYS WHP) during a period of increased surveillance efforts as a result of West Nile virus. Viral entities such as a rotavirus or paramyxovirus had previously been suggested to be the cause, however, in-situ hybridization (ISH) confirmed an orthoreovirus in 2019. In addition, crows were 32 times more likely develop the typical lesions of necrotizing enteritis, typhlocolitis, and fibrous splenic necrosis when the virus was present.2
During a two year period (2016-2017), approximately 20% of crow carcasses examined by the NYS WHP were attributed to reovirosis, which accounted for 70% of all recorded deaths during the winter months, almost exclusively during December and January. Possible explanations for seasonality include immunosuppression due to harsh climate and increased exposure as the result of overcrowding situations during winter roosting.2
Orthoreovirus is one of nine genera under the Spinareovirinae subfamily within family Reoviridae. Other notable Reoviridae genera include Orbivirus (bluetongue and African horse sickness viruses) and Rotavirus.6 Members of the Reoviridae family are double stranded RNA viruses composed of isometric virons measuring 60-80 nm in diameter with an inner protein coat and one or two distinct icosahedral capsids.7 All replicate within the host cell cytoplasm, producing
characteristic paracrystalline arrays of progeny virons. Virons initially attach to the cell via sialic acid receptors on the cell membrane and subsequently undergo receptor-mediated endocytosis via engulfment and internalization of clathrin-coated pits. These newly formed vacuoles then fuse with lysosomes, resulting in the partial digestion of the viron's outer shell. Disruption of the outer shell subsequently initiates significant changes in the supramolecular structure of remaining viral components and also activates the transcriptase, resulting in the transcription of mRNA using released subviral particles in the cytoplasm.7
The intracytoplasmic, golden-brown, birefringent pigment within splenic macrophages is predominantly negative when stained with Perls' Prussian blue (iron), which is consistent with hemozoin. The moderator discussed the genesis of this pigment and its association with Plasmodium and Haemoproteus spp. Parasites in the family Plasmodidae consume up to 80% of an erythrocyte's hemoglobin and subsequently produce a toxic pro-oxidant intermediate, α-hematin. The parasite then converts α-hematin to insoluable and inert β-hematin crystals (hemozoin) in a process known as biocrystallization. Similar pigment associated with blood feeding nematodes is also commonly referred to as "hemozoin" although the crystalline structure differs.
References:
1. Ayalew LE, Ahmed KA, Mekuria ZH, et al. The dynamics of molecular evolution of emerging avian reoviruses through accumulation of point mutations and genetic re-assortment. Virus Evol. 2020 Jan;6:veaa025.
2. Forzán MJ, Renshaw RW, Bunting EM, et al. A NOVEL ORTHOREOVIRUS ASSOCIATED WITH EPIZOOTIC NECROTIZING ENTERITIS AND SPLENIC NECROSIS IN AMERICAN CROWS (CORVUS BRACHYRHYNCHOS). J Wildl Dis. 2019 Oct;55:812?822.
3. Hollmen T, Docherty DE. Orthoreoviruses. In: Thomas NJ, Hunter DB, Atkinson CT, ed. Infectious Diseases of Wild Birds. Ames, IA: Blackwell Publishing; 2007:177.
4. Kant A, Balk F, Born L, et al. Classification of Dutch and German avian reoviruses by sequencing the sigma C protein. Vet Res. 2003 Mar;34:203?212.
5. Lawson B, Dastjerdi A, Shah S, et al. Mortality associated with avian reovirus infection in a free-living magpie (Pica pica) in Great Britain. BMC Vet Res. 2015 Feb 7;11:20.
6. Schoch CL, et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford). 2020: baaa062.
7. Urbano P, Urbano FG. The Reoviridae family. Comp Immunol Microbiol Infect Dis. 1994;17(3-4):151-161.