WSC 2023-2024 Conference 2, Case 2
Signalment:
3-year-old, male neutered domestic short hair cat, feline (Felis catus)
History:
This animal was referred to University hospital with a 5-month history of progressive ataxia and a 5-day history of lethargy. Neurological abnormalities included obtundation and profound cerebellovestibular ataxia of all limbs. Proprioceptive positioning was diffusely delayed, hopping was markedly reduced to absent, and extensor postural thrust was absent. There was also positional vertical nystagmus. MRI scan revealed abnormal areas of hyperintensity in the cerebellum along with cerebellar herniation.
Gross Pathology:
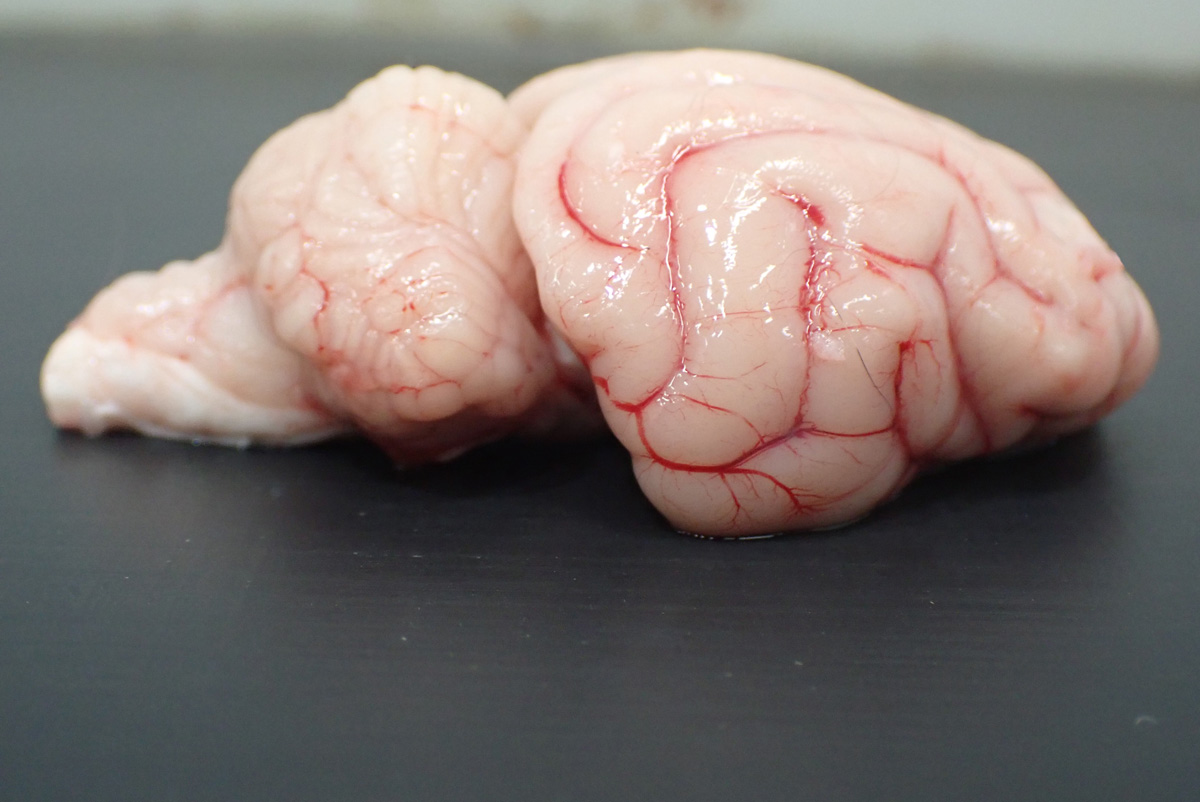
The cerebellum is enlarged with ‘coning’ of the vermis. The cerebral gyri are flattened and widened. On sectioning, the cerebellar white matter was markedly expanded. Thoracic, abdominal, and pelvic organs are unremarkable.
Laboratory Results:
Serology for Toxoplasma gondii was negative.
Microscopic Description:
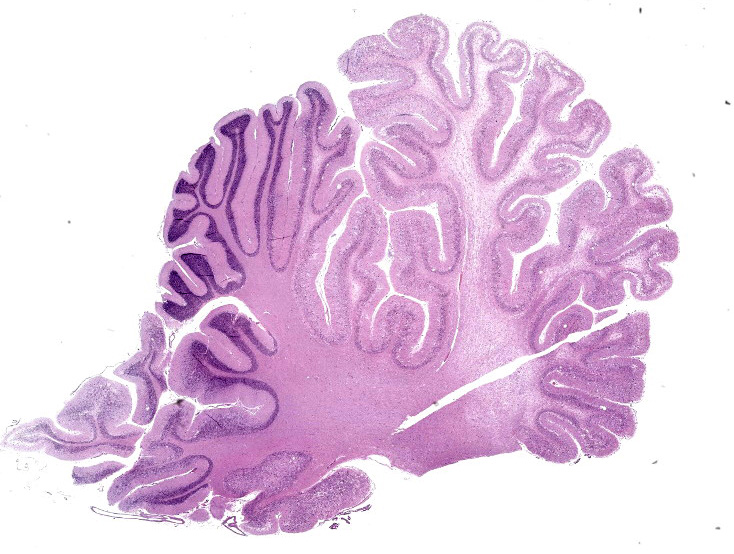
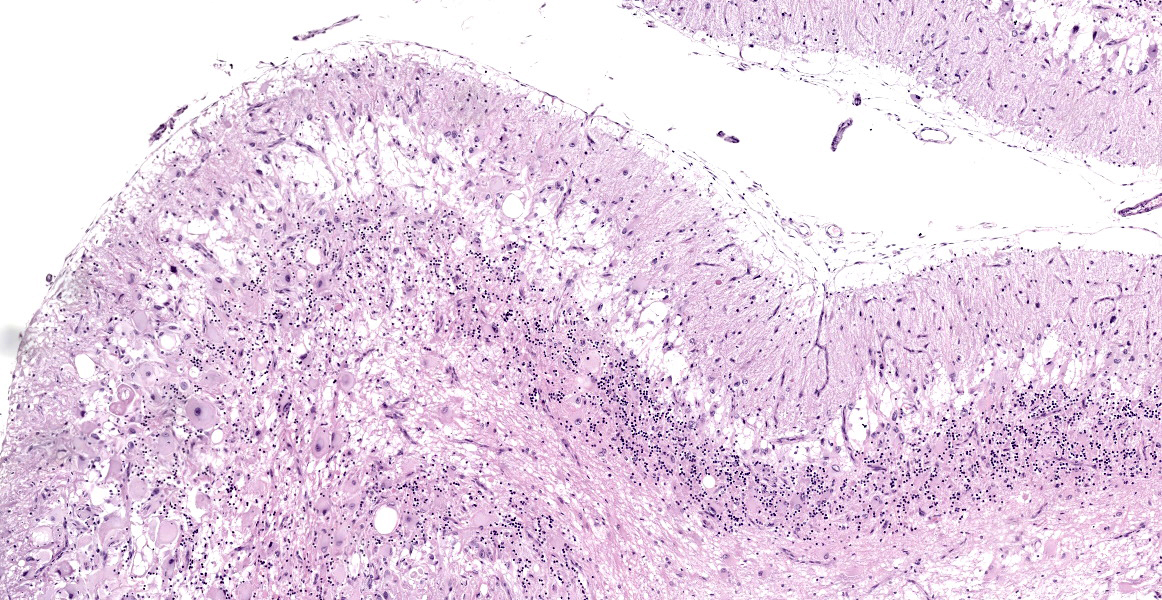
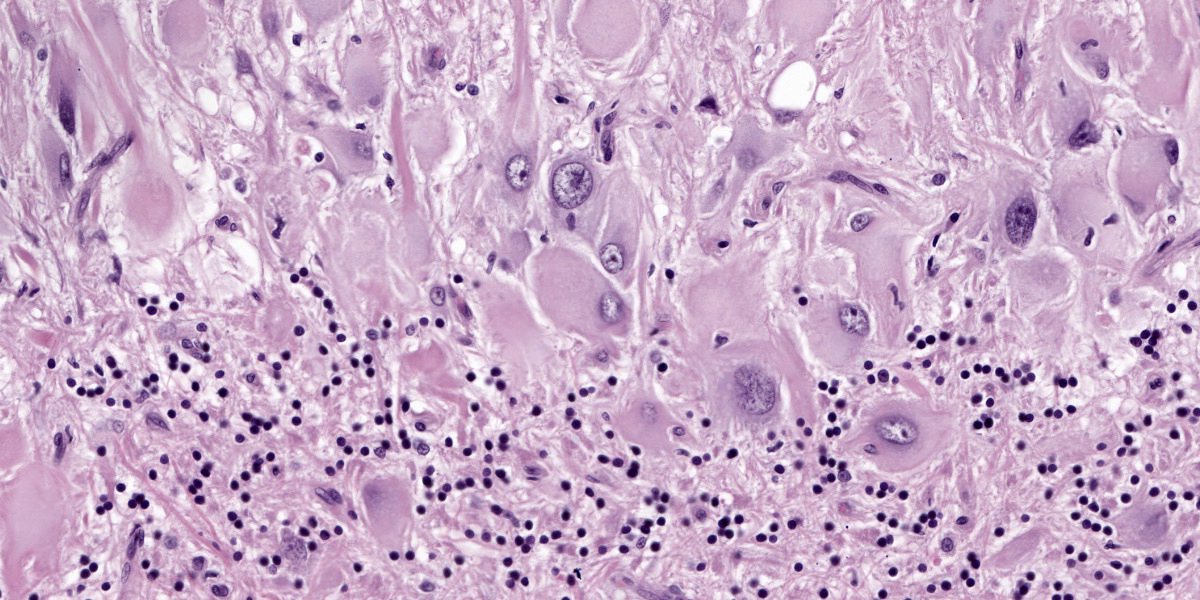
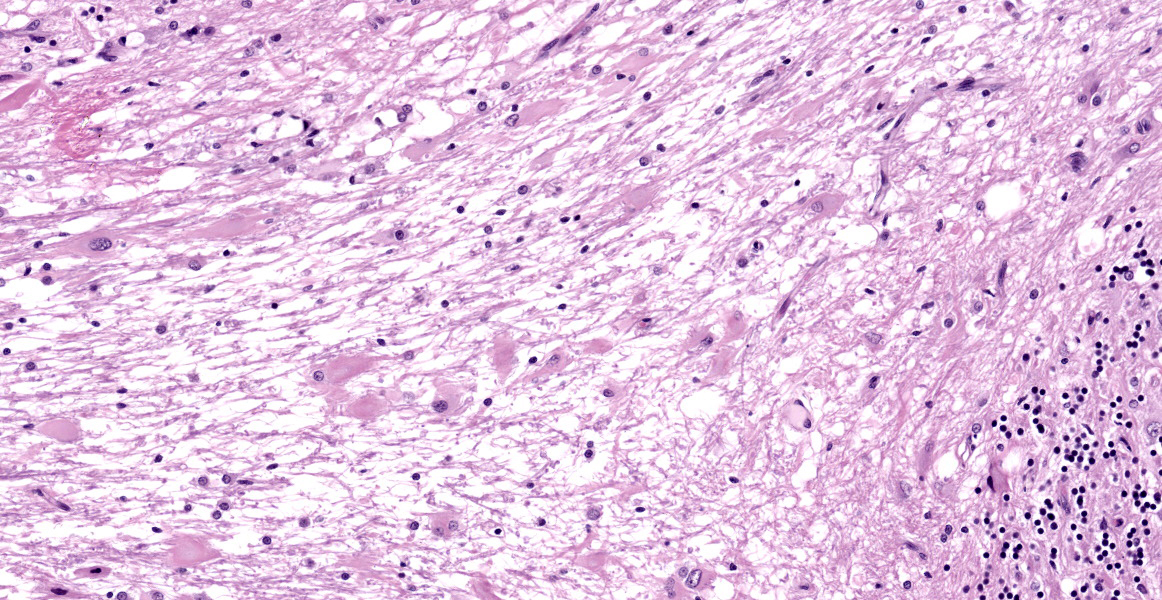
Numerous large dysplastic neurons up to approximately 60 μm in diameter populate the cerebellar Purkinje cell layer and extend into the molecular and granular layers. These pleomorphic cells are characterised by scant to abundant pale to brightly eosinophilic cytoplasm. Their nuclei are occasionally eccentrically located and up to approximately 30 μm in size varying from round to oval to reniform in shape with vesicular chromatin and a prominent nucleolus. In affected areas, the Purkinje cells are shrunken or lost and there is variable marked depletion of neurons in the granular layer. The neuropil is frequently rarefied or finely vacuolated (oedema) and contains numerous, variably-sized swollen axons (spheroids). These severe changes frequently alternate with sharply defined areas where the normal cerebellar architecture is maintained. The white matter is extensively rarefied and contains large numbers of gemistocytes. Examination with a Luxol fast blue stain confirmed marked loss of myelin in affected areas while no change in myelination was found in the cerebellar molecular layer. The meninges and perivascular spaces were multifocally infiltrated by low numbers of lymphocytes. Affected regions of cerebellum were immunohistochemically labeled for GFAP (astrocytes), NeuN (neuronal nuclei), NF clone 52 (axonal neurofilament H;
heavy), and NF clone 312 (axonal pan-neurofilament). Immunohistochemical labeling of PTEN was also carried out to investigate the possibility of loss of PTEN protein expression as a result of PTEN gene mutation. There was consistent cell-specific labeling with each of these five antibodies. While immunolabeling with GFAP or NeuN was not found in dysplastic cells, axonal immunolabeling of NF52 and NF312 was maintained in their axons. Dysplastic neurons presented a heterogenous labeling pattern for PTEN, with the vast majority showing the absence of nuclear and reduced cytoplasmic immunolabeling. There were no abnormal findings in other brain regions.
Contributor’s Morphologic Diagnosis:
Brain: Dysplastic cerebellar gangliocytoma.
Contributor’s Comment:
This is the first report of dysplastic gangliocytoma of the cerebellum in a cat that had presented with neurological deficits indicating cerebello-vestibular system disease. Dysplastic gangliocytoma of the cerebellum, commonly known as Lhermitte-Duclos disease, is an infrequent benign tumour described in human patients.9 The neoplasm usually presents as a single unilateral discrete mass in the cerebellum, and bilateral involvement is very rare.3,4,10,17 There is debate as to whether dysplastic gangliocytoma of the cerebellum represents a true neoplasm or a hamartoma.14 Hamartomas are disorganised masses of normal to dysplastic cells which arise at sites where these cells are normally present.8 The fact that these lesions frequently originate from clonal chromosomal aberrations makes their differentiation from benign tumours challenging and perhaps arbitrary. The World Health Organization (WHO) central nervous system conference has graded dysplastic gangliocytoma of the cerebellum as a grade 1 neoplasia.13 The classic macroscopic lesion is a unilateral enlargement of the cerebellum with maintenance of the folia.3,10,17 Microscopic findings are variable and include replacement of the cerebellar granular layer by large, well-differentiated, dysplastic ganglion cells.4 Dysplastic gangliocytoma of the cerebellum can occur sporadically, but has also been associated with Cowden syndrome, an autosomal dominant genetic disorder characterised by multiple hamartomas that leads to an increased risk of developing benign and malignant tumors. Cowden syndrome is associated with germline mutations of the phosphatase and tensin homolog (PTEN) gene, a tumour suppressor gene that leads to activation of the PI3K/AKT signaling pathway and uncontrolled cell proliferation.8
In human patients the lesion is defined by the following components: (1) variable replacement of the cerebellar internal granular layer by dysplastic ganglion cells, (2) abnormal myelinisation of the molecular layer, (3) reduced number of Purkinje cells, (4) large, bizarre neurons, and (5) vacuolisation of cerebellar white matter.4 Each of these criteria were met in the current case except for abnormal myelinisation of the molecular layer. Interestingly, the current case posed a bilateral presentation, which is rarely described in human patients.3,10,17 The mild inflammation, not considered to be of clinical significance, most likely occurred secondary to decreased cerebrospinal flow as a result of cerebellar enlargement and herniation.5 Immunohistochemical labeling confirmed that the dysplastic cells were of neuronal origin; axonal neurofilament immunolabeling was present, although NeuN immunolabeling was absent. Similar results have been reported in gangliocytomas in human patients.15
Cowden syndrome is a multiple hamartoma syndrome associated with PTEN mutation in 80% of cases of dysplastic gangliocytoma of the cerebellum in human patients, and clinical diagnosis is made by the presence of a combination of characteristic criteria including mucocutaneous lesions and a wide variety of benign and malignant tumors.4 Major and minor diagnostic criteria for Cowden syndrome in humans include adult onset of dysplastic gangliocytoma of the cerebellum and benign lipomas.6,10 A single case of Cowden-like syndrome has been described in the veterinary literature involving a Great Dane puppy that had colorectal hamartomatous polyposis, ganglioneuromatosis, and an associated PTEN mutation.2 PTEN mutation resulting in downregulation/dysfunction has been reported and is suspected to contribute to tumorigenesis in cats and dogs.12,16 In the current case, on IHC for PTEN mutations, the majority of dysplastic neurons showed loss of nuclear PTEN immunolabeling and reduced cytoplasmic immunolabeling. This may suggest that a PTEN mutation contributed to tumourigenesis in this case similar to what has been reported in Cowden syndrome. Although the ‘gold standard’ diagnosis of germline mutations like PTEN has been Sanger sequencing, studies have suggested the possibility of IHC detection of PTEN mutations as a superior approach as this technique also addresses potential epigenetic elements contributing to the loss of PTEN function.7
Contributing Institution:
University College Dublin
School of Veterinary Medicine
Belfield, Dublin 4, Ireland
http://www.ucd.ie/vetmed/
JPC Diagnosis:
Cerebellum: Dysplastic gangliocytoma.
JPC Comment:
The uncontrolled growth that characterizes cancer is effected through two broad mechanisms: gain-of-function mutations in normal proteins that promote cell growth, or loss-of-function mutations that diminish the efficacy of cell growth inhibitors. The former mutations turn normal genes into oncogenes that drive cell proliferation via the production of constitutively active oncoproteins.11 Mutations of the latter type occur in tumor suppressor genes, leading to failure of growth inhibition, a fundamental hallmark of carcinogenesis.11
PTEN mutation, highlighted by the contributor as a critical factor in the development of many dysplastic gangliocytomas, is an example of a loss of function mutation in a tumor suppressor. PTEN is a participant in a canonical cell growth pathway that begins with a receptor tyrosine kinase (RTK). RTKs are transmembrane proteins with an extracellular growth factor binding domain and a cytoplasmic tyrosine kinase domain. In normal signal transduction, an extracellular growth factor binds the RTK’s extracellular binding domain, transiently activating the RTK and causing it to dimerize and autophosphorylate tyrosine residues on its cytoplasmic tail. These phosphorylated tyrosine residues serve as binding and activation sites for cytoplasmic signaling molecules, most notably RAS.11 In the activated state, RAS stimulates two downstream signaling cascades: the MAPK cascade and the PI3K/AKT pathway. The common endpoint of both pathways is the activation or production of transcription factors that move to the nucleus and increase production of proteins that drive progression through the cell cycle. Many proteins within these pathways are encoded by proto-oncogenes; in fact, gain of function point mutations in RAS that cause constant activation of downstream, pro-growth pathways are the most common proto-oncogene abnormalities in human tumors.11
In this exuberant signaling milieu, PTEN’s function is to inhibit PI3K, the first of a series of serine/threonine kinases in the PI3K/AKT pro-growth pathway. Regulation at this stage is critical as AKT, the next kinase in the signaling cascade, phosphorylates more than 150 proteins involved in regulating protein synthesis and apoptosis.11 If PTEN acquires a loss of function mutation, as is the case in up to 80% of dysplastic cerebellar gangliocytomas, this cellular brake is lost, leading to unchecked, pro-growth downstream signaling and a predisposition to tumor development.9
The contributor alludes to the debate over the classification of this entity. A hamartoma, as stated above, is a non-neoplastic growth characterized by an abnormal growth of cells in their normal anatomic location. Some sources distinguish hamartomas from neoplasia by pathogenesis; hamartoma is usually the result of a systemic genetic abnormality while neoplasia tends to be monoclonal since it originates from a single transformed cell.1 In this case, the eye-catching dysplastic neurons are in their native location and possibly arise from a germline mutation in PTEN, making classification as a hamartoma seem appropriate. As noted above, however, the human lesion is described by the WHO as a grade 1 neoplasm, and the literature includes a variety of terminology, including the term “hamartomatous neoplasm,” which provides little clarity.
Conference participants engaged this debate without reaching general consensus, though most participants felt this entity more likely represented a hamartoma. No matter the terminology, the biological behavior of this entity is benign, though significant morbidity can accompany its growth within the confines of the skull.
References:
- Batsakis JG. Nomenclature of developmental tumors. Ann Otol Rhino Laryngol. 1984;93(1):98-99.
- Bemelmans I, Küry S, Albaric O, et al. Colorectal hamartomatous polyposis and ganglioneuromatosis in a dog. Vet Pathol. 2011;48(5):1012–1015.
- Borni M, Kammoun B, Kolsi F, et al. The Lhermitte-Duclos disease: a rarebilateral cerebellar location of a rare pathology. Pan Afr Med J. 2019;33:118.
- Brat DJ, Perry A. Pattern Recognition Series: Practical Surgical Neuropathology: A Diagnostic Approach. 2nd ed. Elsevier; 2018.
- Deren KE, Packer M, Forsyth J, et al. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol. 2010;226(1): 110–119.
- Derrey S, Proust F, Debono B, et al. Association between Cowden syndrome and Lhermitte-Duclos disease. Surg Neurol. 2004;61(5):447–454.
- Djordjevic B, Hennessy BT, Li J, et al. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing.Mod Pathol. 2012;25(5):699–708.
- Hargis AM, Myers S. The Integument. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. Elsevier; 2017:1009.
- Joo G, Doumanian J. Radiographic findings of dysplastic cerebellar gangliocytoma (Lhermitte-Duclos Disease) in a woman with Cowden syndrome: a case study and literature review. J Radiol Case Rep. 2020;14(3):1-6.
- Khandpur U, Huntoon K, Smith-Cohn M, et al. Bilateral recurrent dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) in Cowden syndrome: a case report and literature review. World Neurosurg. 2019;127:319-325.
- Kumar V, Abbas AK, Aster JC. Neoplasia. In: Pathologic Basis of Veterinary Disease. 10th ed. Elsevier; 2022: 286-292.
- Levine RA, Forest T, Smith C. Tumor suppressor PTEN is mutated in canineosteosarcoma cell lines and tumors. Vet Pathol. 2002;39(3):372–378.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organizatio classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820.
- Nowak DA, Trost HA. Lhermitte-Duclos disease (dysplastic cerebellar gan-gliocytoma): a malformation, hamartoma or neoplasm? Acta Neurol Scand. 2002; 105(3):137–145.Rainov NG, Holzhausen H-J, Burkert W. Dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease). Clin Neurol Neurosurg. 1995; 97(2):175 -180.
- Ressel L, Millanta F, Caleri E, et al. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Vet Pathol. 2009;46(5):860–868.
- Zak M, Ledbetter M, Maertens P. Infantile Lhermitte-Duclos disease treated successfully with rapamycin. J Child Neurol. 2017;32(3):322–326.