WSC 2023-2024, Conference 17, Case 2
Signalment:
13-year old, intact female Indian origin Rhesus macaque (Macaca mulatta)
History:
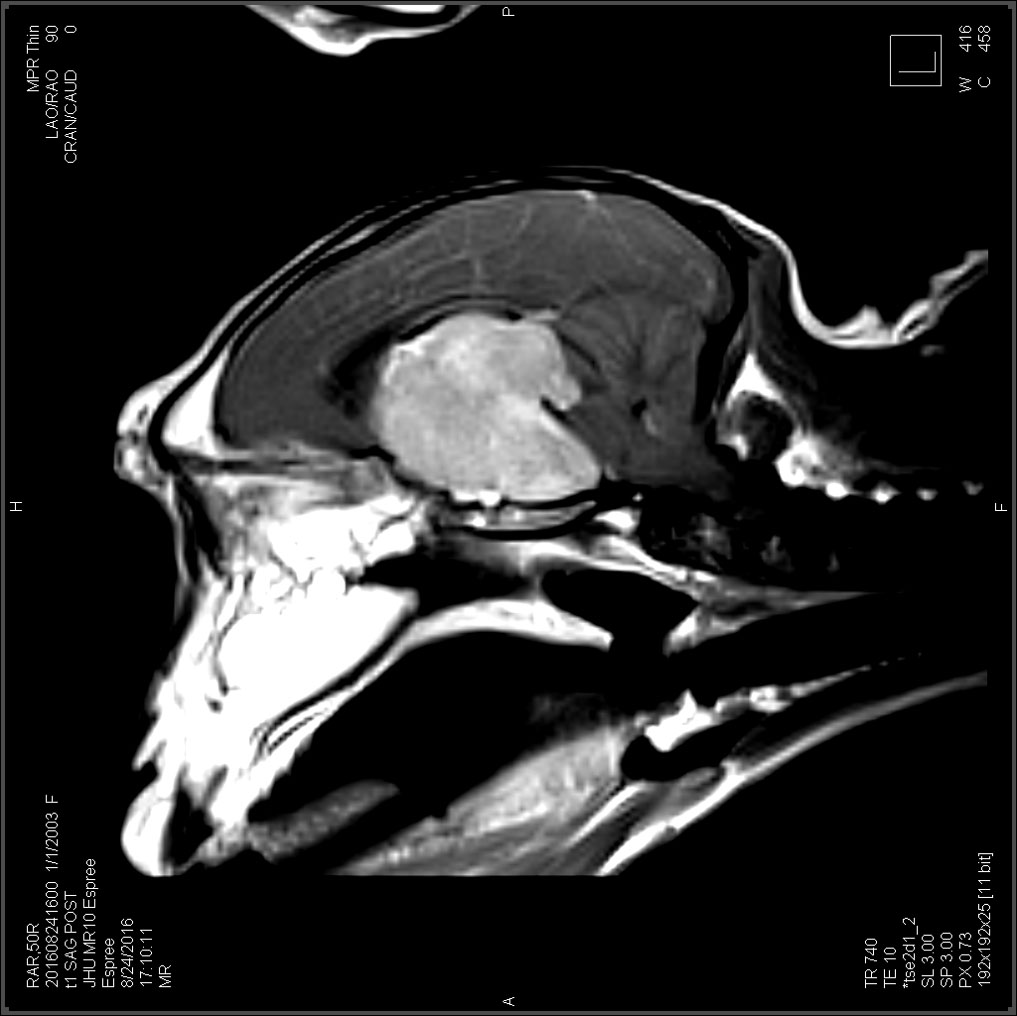
This female Rhesus macaque presented with progressive weight loss, vision loss, and wide circling to the right over the course of one year. She also exhibited galactorrhea for approximately 4 years, despite not having given birth in over two years. Ophthalmologic exam revealed inability to fix or follow an object, searching behavior, and subjective bilateral optic nerve pallor on fundic exam. Subsequent MRI revealed a large (3.2 x 2.9 x 2.5 cm) contrast-enhancing hypophyseal mass protruding
from the pituitary fossa with associated marked compression of the adjacent brain parechyma. Due to poor prognosis, humane euthanasia was elected.
Gross Pathology:
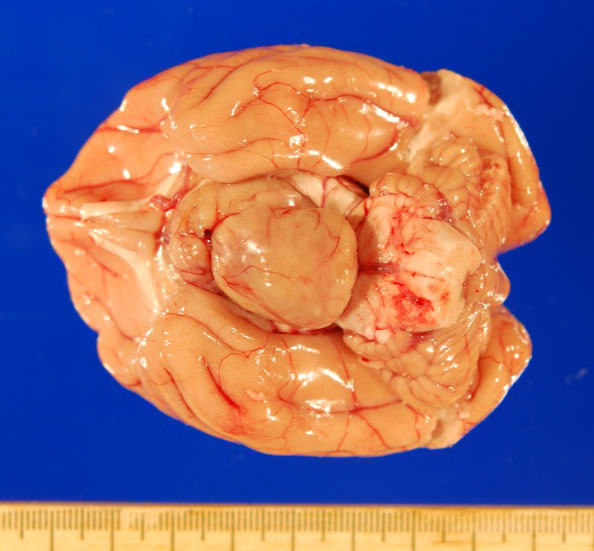
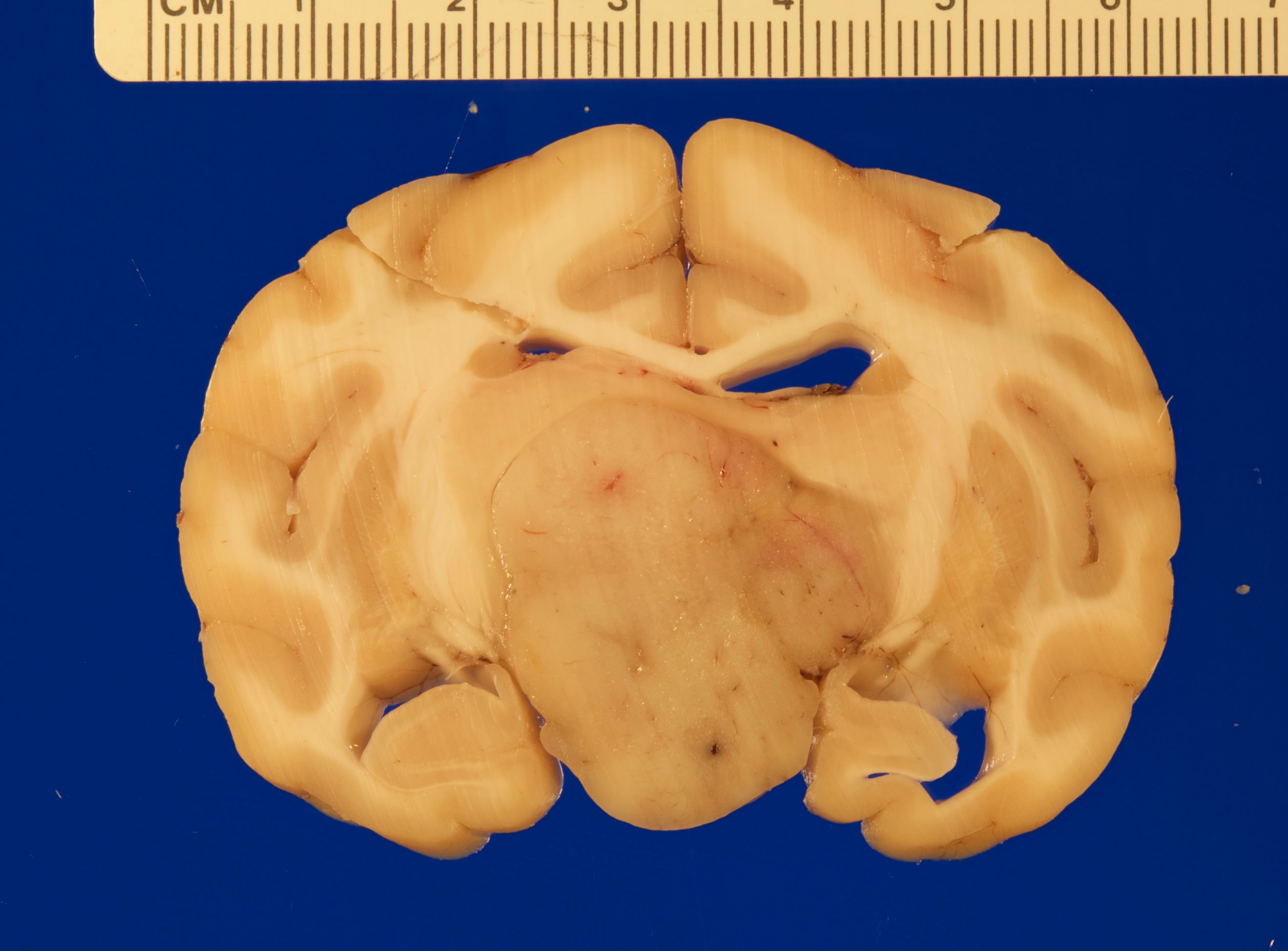
Gross necropsy revealed a 2.4 x 2.0 x 1.4 cm, well-circumscribed, tan, firm mass attached to the mid-ventral aspect of the brain at the level of the pituitary gland dorsal to the sella turcica. The mass compressed adjacent neural tissue and the optic nerve at the level of the optic chiasm. On cut section, the mass extended as far rostrally as the basal ganglia and as far caudally as the pons. The mass also asymmetrically compressed the lateral ventricles, causing mild to moderate hydrocephalus.
Laboratory Results:
Significant clinical pathology results are listed below:
- Complete blood count (CBC): Stress leukogram
- Chemistry panel: Mild hypokalemia
- Thiamine: WNL
- Estrogen (E2): WNL (<5 pg/ml)
- Progesterone (P4): WNL (0.10 ng/ml)
- Prolactin: Elevated (147.6 ng/ml - expected value 20-50 ng/ml)
Immunohistochemistry:
- Synaptophysin (+)
- ACTH (-)
- FSH (-)
- Growth hormone (-)
- MSH (-)
- Prolactin (-)
Microscopic Description:
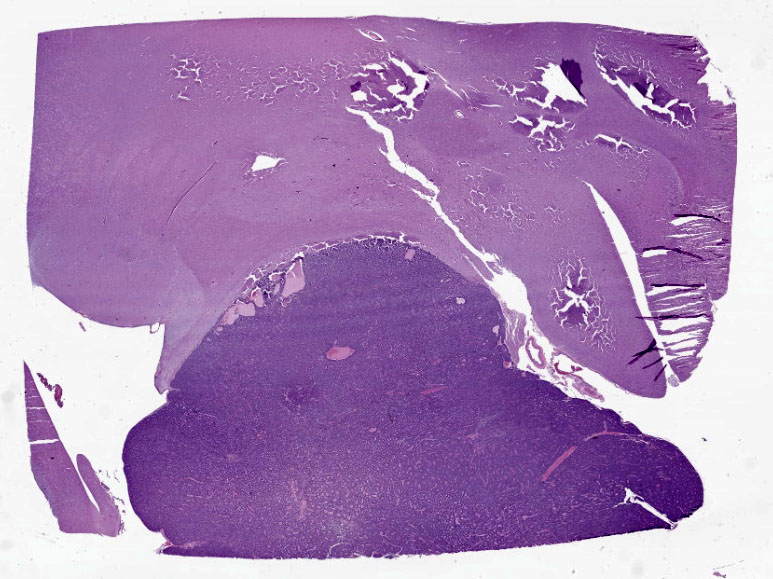
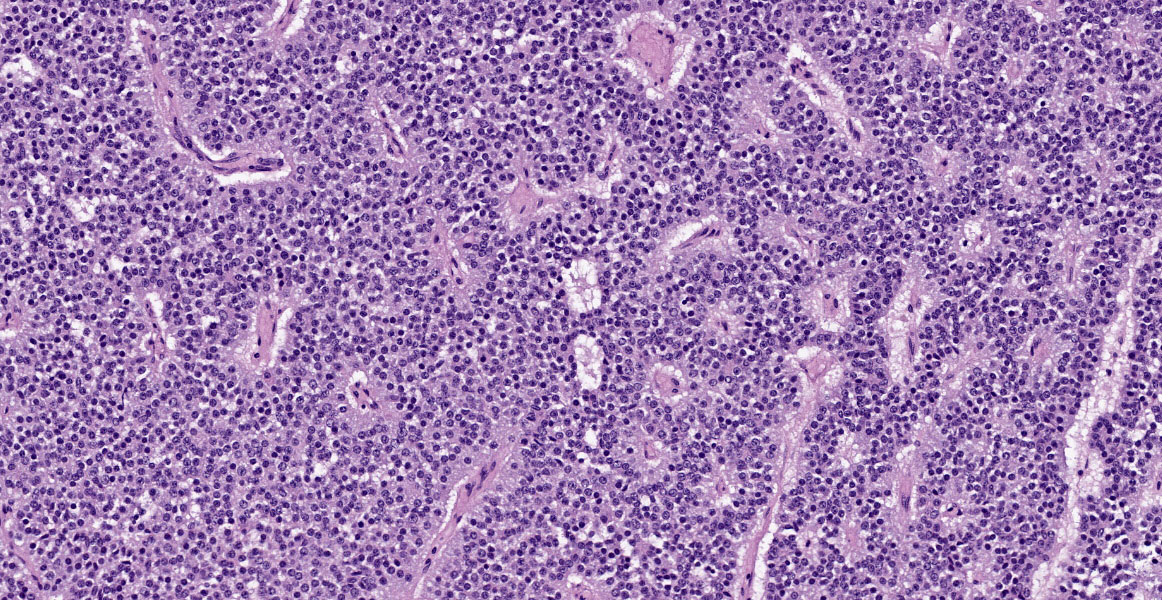
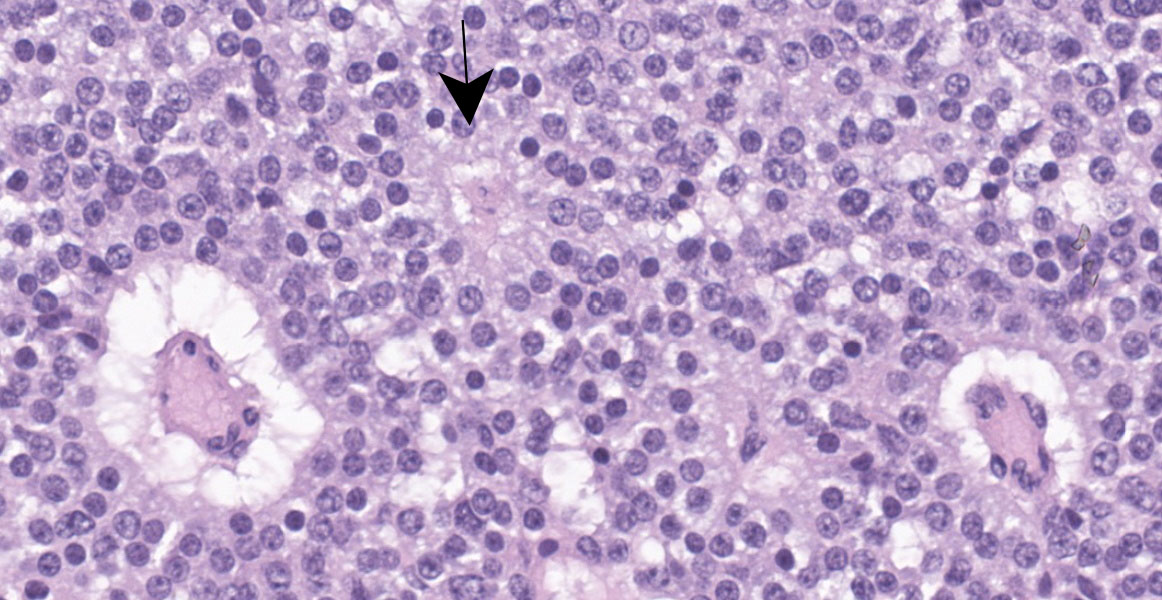
Cerebrum: Expanding and compressing surrounding neuroparenchyma is a densely cellular, well-demarcated, nodular, unencapsulated neoplasm. Neoplastic cells are closely packed and are arranged in nests/packets supported on a fine fibrovascular stroma. Neoplastic cells frequently palisade around blood vessels (pseudorosettes) or a central area of fiber-rich neuropil (Homer-Wright rosettes). Cells are columnar to polygonal with indistinct cell borders and a scant to moderate amount of eosinophilic granular cytoplasm. Nuclei are round and centrally located with coarsely stippled chromatin and 1-2 variably prominent nucleoli. Anisocytosis and anisokaryosis are mild. Mitoses are rare (avg <1 per 10 high powered fields). There are multifocal areas of necrosis within the center of the neoplasm consisting of pyknotic and karyorrhectic cellular debris admixed with concretions of mineral, fibrosis, and rare cholesterol clefts. There is no evidence of vascular or lymphatic invasion. There is diffuse compression and variable rarefaction of the adjacent neuropil. Within the normal adjacent section of brain, there are numerous mineralized blood vessels.
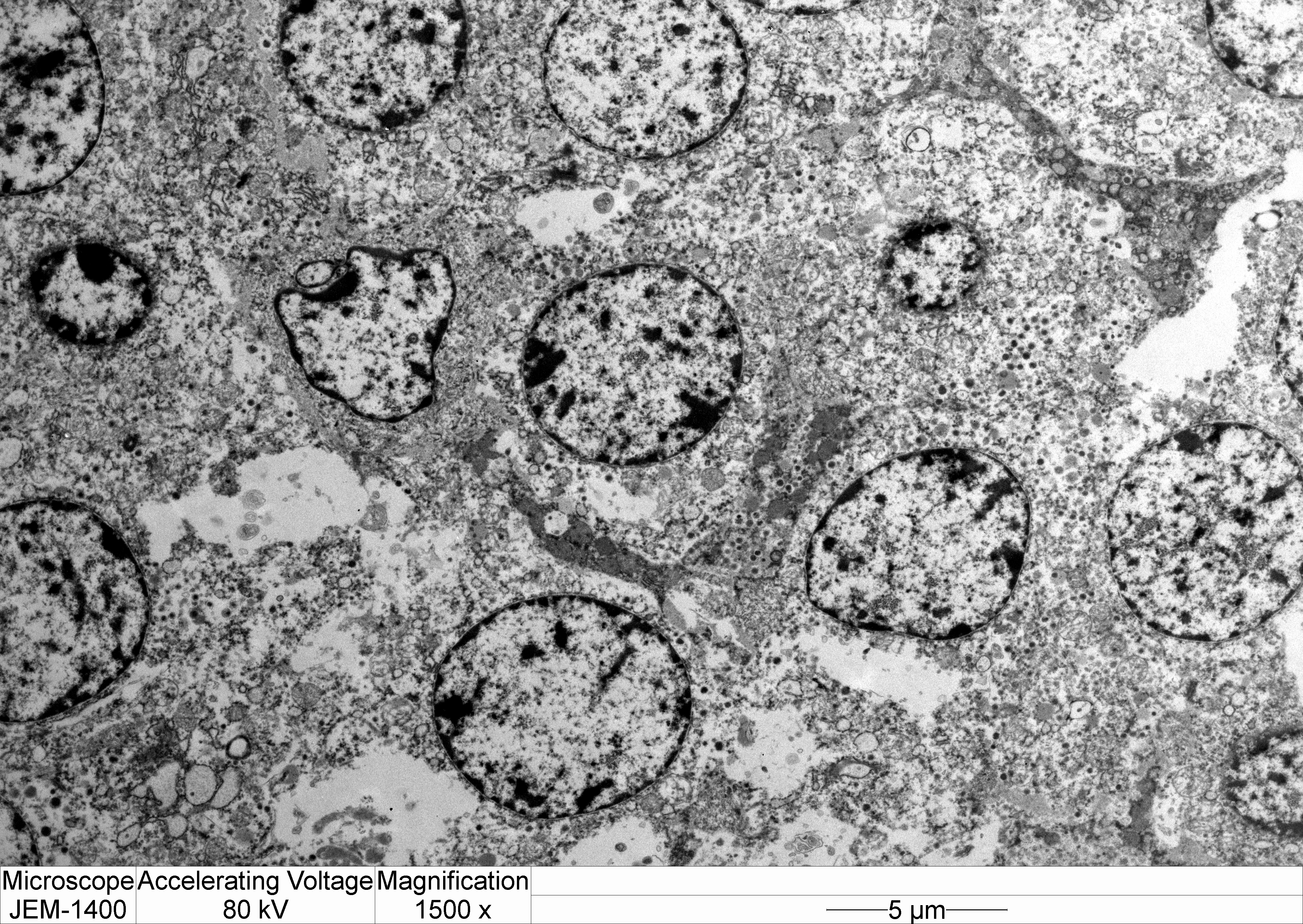
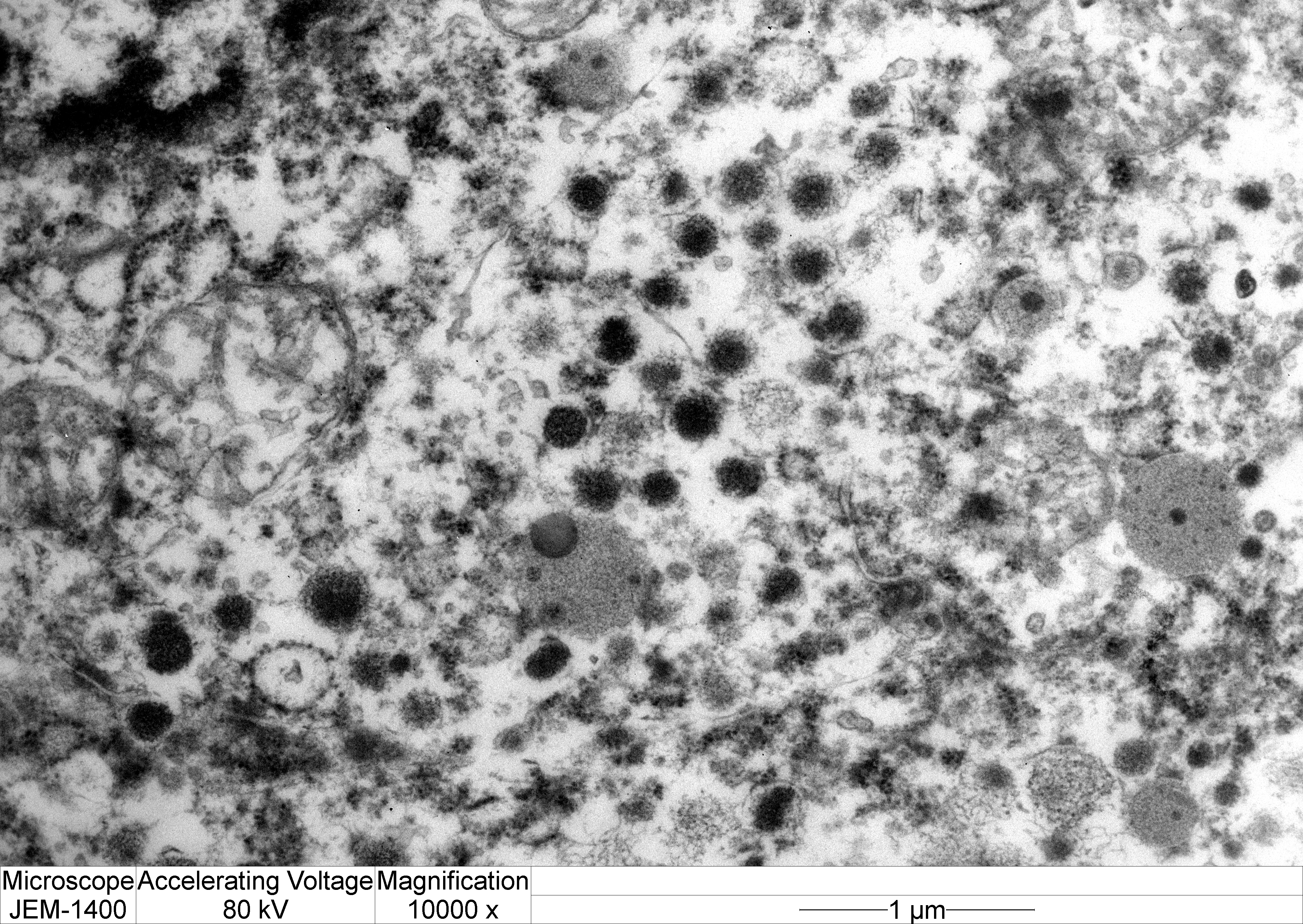
Transmission electron microscopy: Neoplastic cells have indistinct borders. Within the cytoplasm are multiple, approximately 100 nm, electron dense, spherical granules. The Golgi apparatus and endoplasmic reticulum are indistinct and often poorly formed.
Contributor’s Morphologic Diagnosis:
Cerebrum: Pituitary null-cell adenoma.
Contributor’s Comment:
This aged female rhesus macaque presented with progressive weight loss, hyperprolactinemia and galactorrhea without recent pregnancy, and neurologic signs including vision loss and circling. The most significant lesion on necropsy was a large, compressive pituitary adenoma, which is believed to be the main contributing factor for all clinical findings. Immunohistochemistry of the tumor is diffusely negative for hormone production and electron microscopy revealed multiple small, approximately 100 nm granules. Combined, these results highly suggest that this tumor is an endocrinologically inactive (aka, non-functional or null) adenoma.
In veterinary species, non-functional pituitary adenomas occur most commonly in dogs, cats, and parakeets, with the most common type being chromophobe adenomas arising from the pars distalis.12,13 Reports of pituitary adenomas in non-human primates are scattered, but have been noted in both Old World and New World primates.4,5,11 The majority of these tumors are described as lactotroph or corticotroph adenomas.5,11 However, there is one case report of a suspected non-functional adenoma with galactorrhea in a male Rhesus macaque, which describes a similar entity to the present case, both clinically and histologically.4
As these tumors are often behaviorally benign, clinical signs of nonfunctional adenomas are typically due to expansion of the tumor into the unaffected pituitary and adjacent CNS, leading to decreased secretion of pituitary hormones and/or CNS dysfunction.12,13 For instance, progressive weight loss and muscle atrophy occurs due to decreased growth hormone; gonadal atrophy due to decreased gonadotropic hormones; and dilute urine with low specific gravity in the face of dehydration due to decreased antidiuretic hormone. Thus, panhypopituitarism caused by endocrinologically inactive pituitary adenomas should be a differential in older animals with incoordination, depression, polyuria, blindness, and sudden behavioral changes. Additionally, as in this case, animals may develop blindness with fixed, dilated pupils due to dorsal extension of the tumor and compression of the optic nerves. Ophthalmic exam is typically otherwise unremarkable because dysfunction is originating from the CNS.
Typical gross lesions of null cell pituitary adenomas can include the following:
- Large tumor (>1cm) with compression or replacement of the remaining adenohypophysis, infundibular stalk, and hypothalamus;
- Small thyroid glands;
- Small adrenal glands due to cortical atrophy;
- Small gonads due to atrophic seminiferous tubules with little active spermatogenesis; and
- Atrophy of skin and muscle.12,13
Histopathologic and electron microscopic features characteristic of null cell adenomas in humans are elongated small cells forming pseudorosettes around dilated capillaries; modest cytoplasm; poorly developed rough endoplasmic reticulum and Golgi apparatus; and rare, round, small secretory granules (up to 250 nm).7,9,10
Ancillary diagnostics, including immunohistochemistry (IHC) and electron microscopy, are key to diagnosing null cell adenomas. The pituitary gland is made up of at least six different cell types that can give rise to tumors that are clinically functioning or silent. Each of these cell types produce one or more specific hormones that can be targeted via immunohistochemistry (See Table 2).9,10 Moreover, plurihormonal adenomas can also occur. Furthermore, neuroendocrine tumors from other regions of the body may metastasize to the pituitary and thus can be positive for both synaptophysin and chromogranin; therefore, other markers may be necessary for differentiation.2
Electron microscopy can provide important information regarding the intracellular structure and hormonal activity of the cells.9 The size and number of secretory granules as well as the degree of development of organelles such as rough endoplalsmic reticulum (RER) and Golgi apparatus can help indicate the secretory status of tumor cells.8,9 In the present case, the poorly-formed RER and Golgi as well as scant, small granules suggest a non-functional secretory status.8,10
Interestingly, this animal was galactorrheac with elevated serum prolactin levels despite diffuse negative immunoreactivity for prolactin in the tumor. We propose that hyperprolactinemia in this case is due to “stalk syndrome” r “pituitary stalk compression syndrome,” a phenomenon in which non-secretory suprasellar tumors induce hyperprolactinemia by inhibiting dopamine delivery to lactotrophs.1-3 Since dopamine is a prolactin-inhibiting factor, reduction of dopamine levels leads to increased prolactin output. It is hypothesized that dopamine levels are reduced by one of two mechanisms: 1) physical compression of the dopaminergic neurons of the infundibular stalk or 2) disruption of hypophyseal portal blood flow delivering dopamine to lactotrophs.3,7 However, increased compression caused by continued growth of the tumor may eventually lead to lactotroph insufficiency and failure.3
|
Marker |
Tumor Type |
|
GH |
Somatotroph adenomas Mammosomatotroph adenomas |
|
PRL |
Lactotroph adenomas Lactotroph ademonas with GH reactivity |
|
TSH |
Thyrotroph adenomas |
|
ACTH |
Corticotroph ademonas |
|
FSH |
Gonadotroph adenomas |
|
MSH |
Melanotroph adenomas |
|
Table 2. IHC hormone markers for pituitary adenomas. GH: Growth Hormone; PRL: Prolactin; TSH: Thyroid Stimulating Hormone; ACTH: Adrenocorticotropin Hormone; FSH: Follicle Stimulating Hormone; MSH: Melanocyte Stimulating Hormone
|
|
In humans, the degree of prolactinemia can provide some clues to help differentiate a prolactinoma from a large tumor with “stalk syndrome.” Serum prolactinemia over 200 ng/mL in humans is almost always due to a hormone-producing prolactinoma, while less than 200 ng/mL can be associated with stalk effect.6 In the present case, serum prolactin levels measured at 147.6 ng/ml (expected value 20-50 ng/ml). Unfortunately, normal ranges are less established in rhesus macaques, and the relationship of degree of elevation to tumor type remains unclear.
Contributing Institution:
Johns Hopkins University School of Medicine
Department of Molecular and Comparative Pathobiology
http://www.hopkinsmedicine.org/mcp
JPC Diagnosis:
Pituitary gland: Pituitary adenoma.
JPC Comment:
As noted by the contributor, the pituitary gland is classically described as having six different cell types, each of which can become neoplastic. The clinical and biochemical signatures of pituitary adenomas differ depending on the cell or cells of origin and on whether these cells produce their native hormone products.
Hormone production is rarely an all-or-nothing affair in pituitary tumors, and clinical signs referable to these neoplasms therefore exist on a continuum.6 In humans, a large number of pituitary adenomas, 22% - 54% depending on the study, present with signs related to the mass effect of the tumor rather than excess hormone secretion; these tumors are referred to as “clinically nonfunctioning pituitary adenomas,” or NFPAs.6 A related term, the “silent pituitary adenoma,” or SPA, refers to tumors that express transcription factors or their hormone products at a level detectable by immunohistochemistry, but not at a level detected clinically.6 A NFPA can be converted to an SPA if a a clinically silent pituitary adenoma nonetheless, on investigation, shows positive IHC immunoreactivity to relevant hormones or transcription factors. In between these lie the “whispering adenomas,” characterized by elevated serum hormone levels, but borderline, often overlooked clinical symptoms.6
At the extreme end of this secretory spectrum is the rarest pituitary tumor type of them all, the null cell adenoma. Null cell adenoma is a diagnosis of exclusion and requires immunonegativity of all adenohypophyseal hormones and, in humans, a lack of cell lineage-specific transcription factors.6 The distinction is important in humans as null cell adenomas generally have a more aggressive course and more unfavorable outcomes compared with SPAs, including those who produce no hormones, but still express lineage-specific transcription factors.6
Conference discussion of this deceptively complex case began with a review of the anatomy and histology of the pituitary gland and its consistuent parts—the pars distalis, pars intermedia, and pars nervosa—along with an overview of pituitary neoplasia generally. Dr. Miller noted that several pituitary neoplasias have species predilections, including corticotroph adenoma/carcinoma (most common in dogs); pars intermedia adenoma (most common in horses and associated with pituitary pars intermedia dysfunction, or PPID); somatotroph adenoma (most common in cats and budgerigars); and lactotroph adenomas (most common in macaques, rats, and rabbits). Particular attention was given to the equine pars intermedia adenoma as its pathogenesis is unique and results from loss of dopaminergic inhibitory innervation from the hypothalamus, leading to unchecked proliferation of the pars intermedia.
With this background knowledge firmly in hand, conference participants moved to the first hurdle: tissue identification. While most conference participants were able to identify the tissue confidently, a significant minority initially identified this tumor as an embryonal tumor such as medulloblastoma. Dr. Miller noted that a firm grasp of neuroanatomy is key to tissue identification in this slide. Evaluation of the adjacent neuroparenchyma contains a haphazard arrangement of scattered neuronal cell bodies that is characteristic of the thalamus. This location, along with the rich vascularization of and the multifocal aggregates of eosinophilic fluid within the tumor, all suggest pituitary origin.
With the neoplasm localized to the pituitary, Dr. Miller emphasized its well-demarcated, expansile, non-invasive nature, which is apparent on subgross examination. As invasion is the most important histologic feature that differentiates pituitary adenoma from carcinoma, the lack of invasion gives this tumor a fairly classic pituitary adenoma appearance. Finally, Dr. Miller noted the multifocal mineralization in vascular walls throughout the neuroparenchyma in the examined section. This is a common age-related lesion in many animals and, in aged macaques, the thalamus is a common location to find this incidental change.
References:
- Al-Brahim NYY, Asa SL. My approach to pathology of the pituitary gland. J Clin Pathol. 2006;59:1245-1253.
- Asa SL. Practical pituitary pathology: what does the pathologist need to know? Arch Path Lab Med. 2008;132(8):1231-1240.
- Bergsneider M, et al. The pituitary stalk effect: is it a passing phenomenon? J Neurooncol. 2014;117(3):477-84.
- Chalifoux LV, MacKey JJ, King NW. A sparsely granulated, nonsecreting adenoma of the pars intermedia associated with galactorrhea in a male Rhesus Monkey (Macaca mulatta). Vet Pathol. 1983; 20:541-547.
- Daviau JS, Trupkiewicz JG. Pituitary adenoma with galactorrhea in an adult male Cynomologus Macaque (Macaca fascicularis). JAALAS. 2001;40(5):57-59.
- Drummond J, Roncaroli F, Grossman AB, Korbonits M. Clinical and pathological aspects of silent pituitary adenomas. J Clin Endocrinol Metab. 2019;104(7):2473-2489.
- Dulai M, Vogel H. Pituitary adenomas. In: Nervous System. New York, NY: Cambridge University Press; 2009:262-284.
- Horvath E, Kovacs K. Fine structural cytology of the adenohypophysis in rat and man. J Electron Microsc Tech. 1998;8(4): 401-432.
- Kovacs K, Horvath E, Vidal S. Classification of pituitary adenomas. J Neurooncol. 2001;54:121-127.
- Osamura RY, et al. Pathology of the human pituitary adenomas. Histochem Cell Biol. 2008;130:495-507.
- Remick AK, et al. Histologic and immunohistochemical characterization of spontaneous pituitary adenomas in fourteen Cynomolgus Macaques (Macaca fascicularis). Vet Pathol. 2006;43:484-493.
- Rosol TJ, Grone A. Pituitary gland. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Volume 3. Elsevier;2016:276-291.
- Rosol TJ, Meuten DJ. Tumors of the pituitary gland. In: Tumors in Domestic Animals. 5th ed. Wiley-Blackwell;2017:768-782.