WSC 2023-2024, Conference 17, Case 1
Signalment:
1-year-old, male neutered mixed-breed dog (Canis familiaris)
History:
This dog presented for severe cervical pain progressing to C6-T1 myelopathy with paraplegia. An MRI of the cervical region and a CSF analysis were within normal limits. The patient was not responsive to antibiotics, prednisone, or cytarabine treatment. The owner elected euthanasia, and the body was submitted for necropsy.
Gross Pathology:
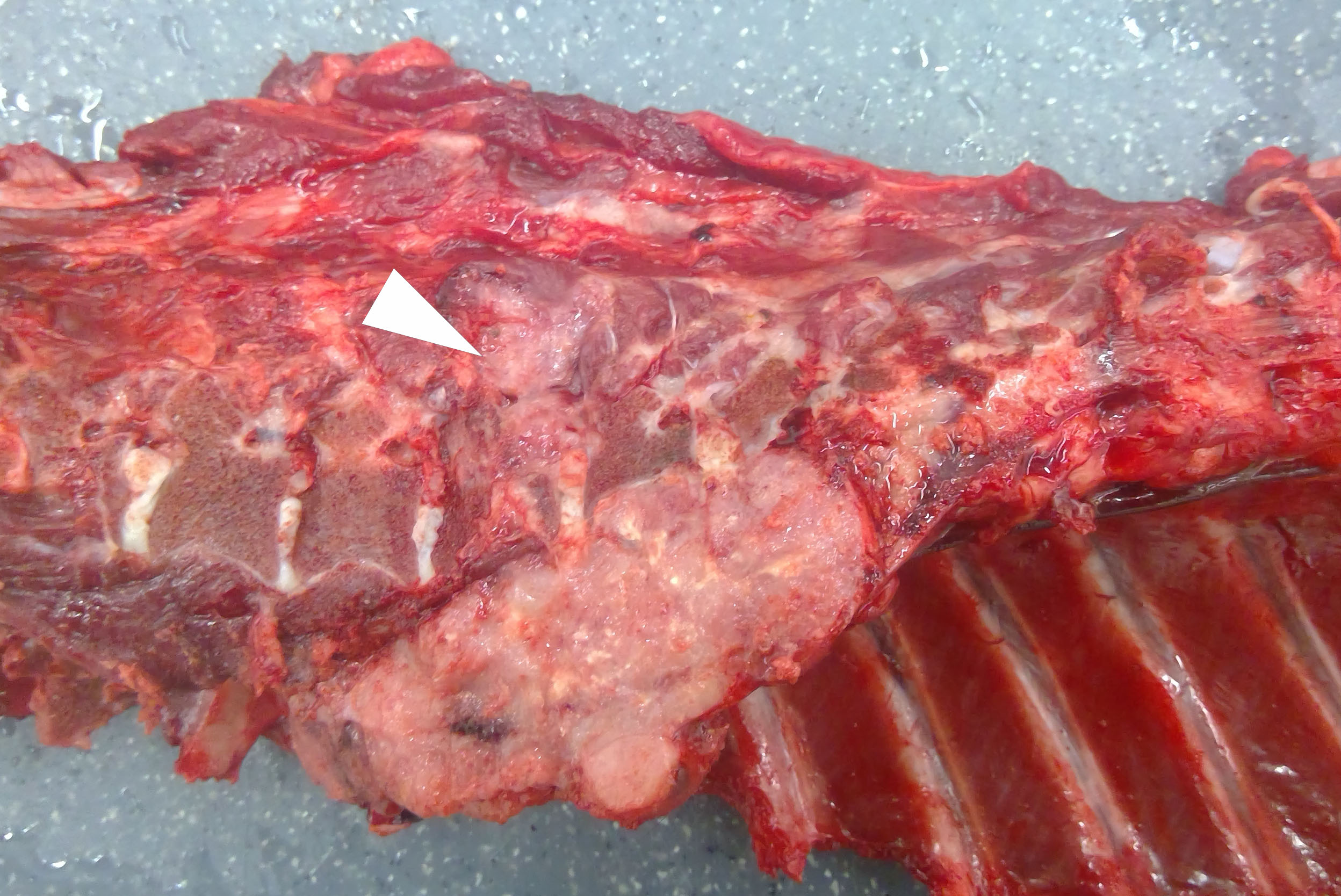
The gross examination was unremarkable except for a 12 x 6 x 6 cm, firm, tan, multilobulated mass in the posterior mediastinum adjacent to the bodies of vertebrae T5-T7. The mass extended through the intervertebral foramen into the vertebral canal, compressed the spinal cord at the level of T6, and infiltrated the adjacent epaxial skeletal muscle.
Microscopic Description:
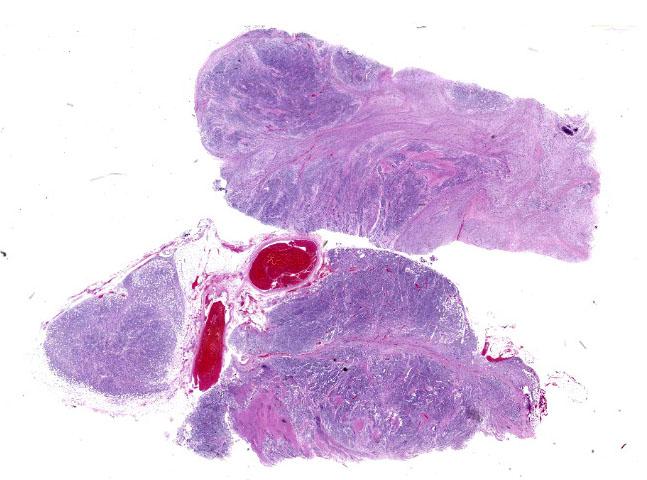
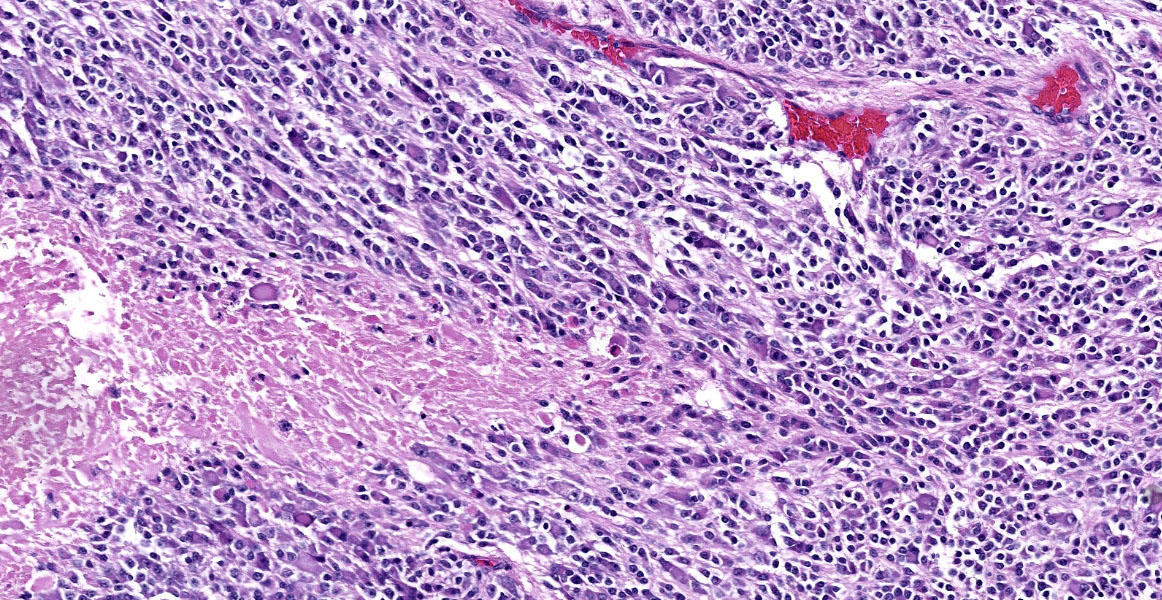
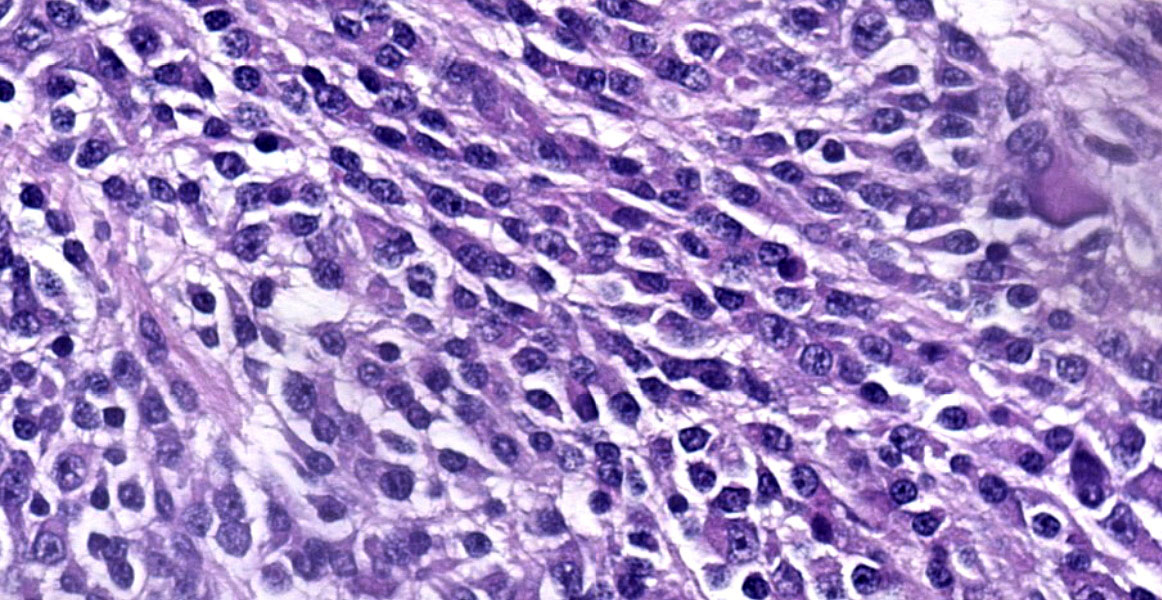
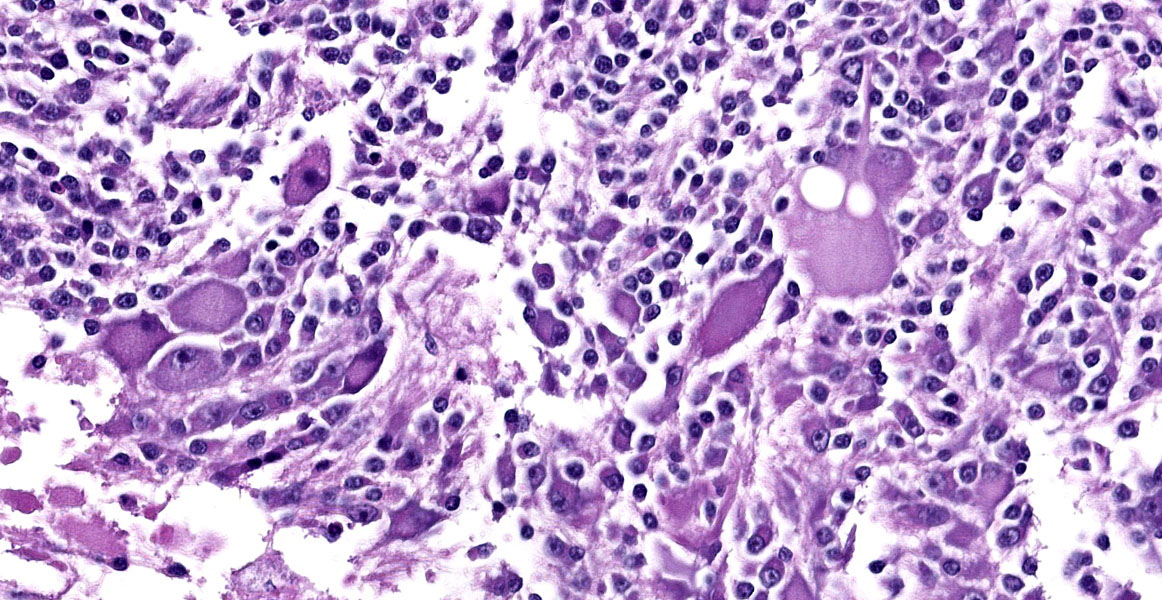
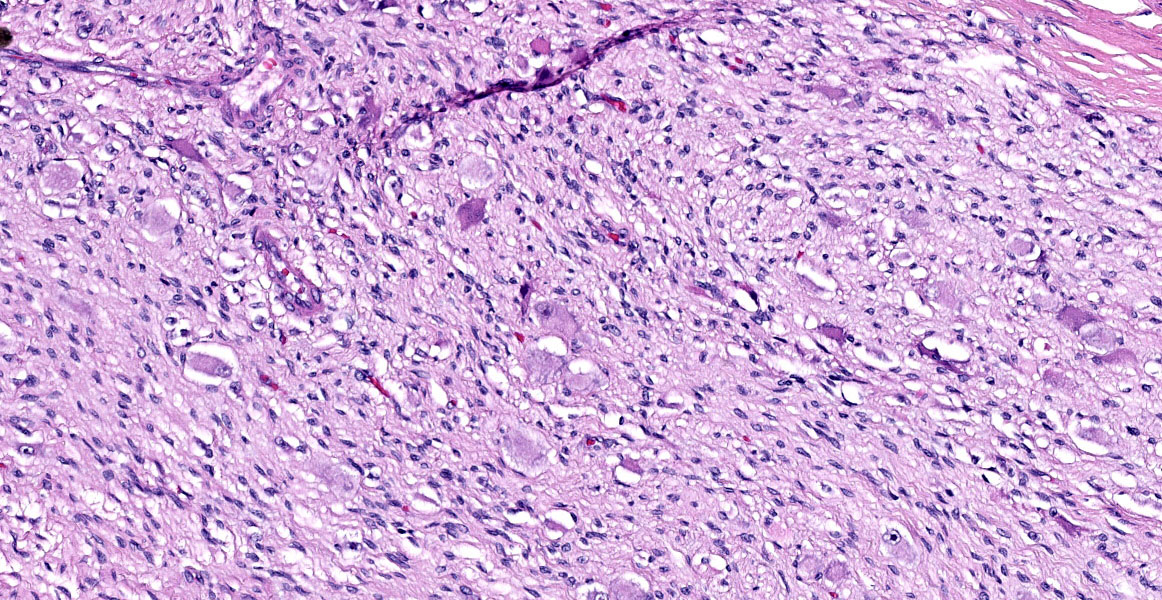
The mass is composed of lobules of round to polygonal neoplastic cells separated by thick bands of connective tissue. The cells show considerable variation in size, ranging from 15µm to over 100µm in diameter. The small cells are round with scant eosinophilic cytoplasm and round nuclei with coarsely clumpedchromatin and indistinct nucleoli (neuroblasts). The larger cells have abundant granular cytoplasm which is eosinophilic around the nucleus and basophilic at the periphery. Nuclei in these cells are round and usually eccentric with vesicular chromatin and single, large, central nucleoli (ganglion cells). The larger cells are often dissected by streams of spindle cells and wavy fibrillar to vacuolated eosinophilic material (Schwannian stroma). Anisocytosis and anisokaryosis are marked and the mitotic rate is higher in the smaller cells (15 in 2.37 mm2) than in the larger cells (2 in 2.37 mm2). In approximately 25% of the mass, the cells are hypereosinophilic with loss of cellular detail (coagulation necrosis), and a focal area is replaced by granular basophilic material (mineralization). At the periphery of the mass, the neoplastic cells infiltrate the adjacent adipose tissue.
Contributor’s Morphologic Diagnosis:
Posterior mediastinal mass: Ganglioneuroblastoma.
Contributor’s Comment:
Peripheral neuroblastic tumors arise from immature cells in the ganglia of the cranial and spinal nerves and the sympathetic nervous system. Locations include the adrenal gland, neck, mediastinum, retroperitoneal space, and pelvis. These tumors are classified based on the degree of differentiation: neuroblastomas are composed of small, undifferentiated cells (neuroblasts); ganglioneuroblastomas also contain neuroblasts, along with maturing and mature ganglion cells, nerve fibers, Schwann cells and stroma (Schwannian stroma); and ganglioneuromas, the most differentiated of the three, are composed of mature ganglion cells, nerve fibers, Schwann cells, and Sch-wannian stroma. Ganglioneuroblastomas are further classified, based on the amount of Schwannian stroma, into intermixed (Sch-wannian stroma-rich) and nodular (Schwannian-stroma rich and Schwannian stroma-poor areas) subtypes. The presence of neuroblasts, gangliocytes, nerve fibers and Schwannian stroma in this case is consistent with a gangiolneuroblastoma.20 Based on the human classification system, this tumor could be further characterized as a nodular subtype.
Neuroblastomas, ganglioneuroblastomas, and ganglioneuromas can also arise from neuronal progenitors in the central nervous system (central neuroblastic tumors) and the olfactory epithelium (olfactory neuroblastic tumors). Olfactory neuroblastomas are also known as esthesioneuroblastomas. Ganglioneuromatosis is a condition characterized by nodular proliferation of neural tissue in the intestine with a segmental or diffuse distribution.
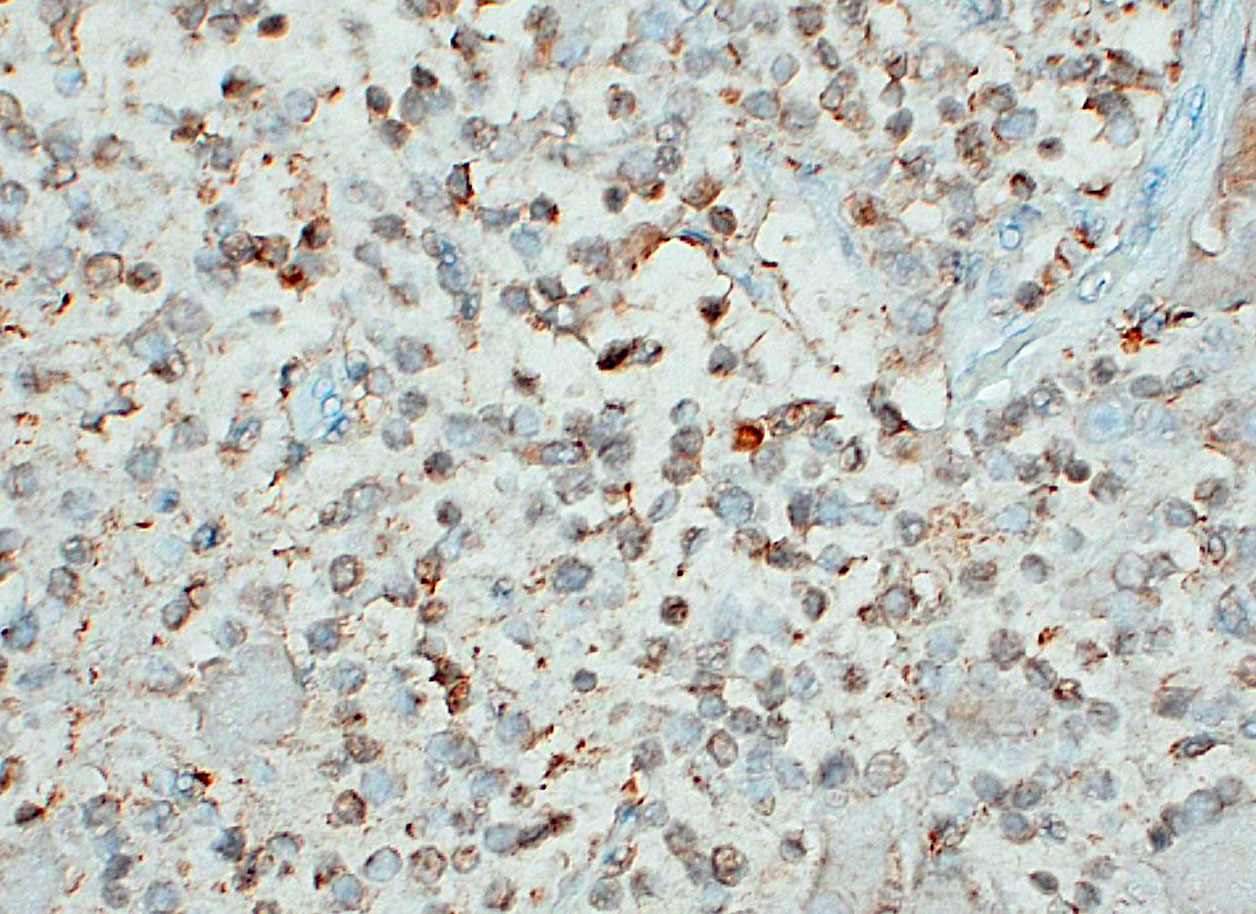
Ganglioneuromatosis is classified as a hamartomatous rather than neoplastic process and is associated with genetic syndromes, such as phosphatase and tensin homolog hamartoma (PTEN) syndrome, neurofibromatosis type 1 or 2, and multiple endocrine neoplasia type IIb.9 By immunohistochemistry, neuroblasts are positive for synaptophysin, neuron specific enolase (NSE), and chromogranin A, although immunoreactivity in this population may be weak or variable; ganglion cells and neural processes are positive for neurofilament and NSE; and Schwann cells are positive for S100, NSE, and GFAP.3,7,10 In humans, neuroblastic tumors comprise 80% of neoplasms in children under 5 years old and are rare after the age of 10.1 Prognosis depends on tumor type, and cellular features (degree of differentiation, mitotic rate, karyorrhexis).19
In general, ganglioneuromas develop in older children and adults and are considered benign with a favorable prognosis. The prognosis for neuroblastomas depends on the age of the patient, the degree of differentiation of the neuroblasts, and the mitosis-karyorrhexis index. Intermixed ganglioneuroblastomas are associated with a favorable prognosis while the prognosis of the nodular subtype depends of the degree of differentiation of the neuroblastic component.
Peripheral neuroblastomas have been documented in dogs3 and cattle11,22 and peripheral ganglioneuromas are described in dogs,5 horses,2 cats,8 and pigs.7 Ganglioneuromatosis has also been reported in a horse,16 dogs,12 and cattle.4 Previous reports of veterinary ganglioneuroblastomas are listed in Table 1.
Contributing Institution:
Virginia-Maryland College of Veterinary Medicine
205 Duck Pond Dr.
Blacksburg VA 24061
https://vetmed.vt.edu/
JPC Diagnosis:
Fibroadipose tissue: Ganglioneuroblastoma.
JPC Comment:
As the contributor notes, peripheral neuroblastic tumors arise from sympathetic nervous system progenitor cells. These progenitor cells migrate from the neural crest to form, among other tissues, sympathetic ganglia and the chromaffin cells of the adrenal medulla.3
|
Species (breed) |
Age/Sex |
Location |
Clinical Outcome |
|
Dog (Bichon-frise)10 |
13 years/MN |
Oral cavity |
Died of heart failure 2 months after surgery |
|
Dog (Lab)18 |
18 months/M |
Cranial mediastinum |
Euthanized 1 week after diagnosis |
|
Dog (German Shepherd)17 |
8 years/M |
Footpad |
Amputation with no recurrence for over 1 year |
|
Cat (mixed)15 |
11 month/MN |
Facial nerve |
Died 30 days after onset of neurologic signs |
|
Cat (DSH)21 |
8 years/MN |
Footpad |
Treated with electrochemotherapy; remission for more than 1 year |
|
Sheep (Akkaraman)23 |
18 months/not specified |
Retroperitoneum |
Diagnosed at slaughter |
|
Calf (Japanese Black)13 |
Newborn/M |
Cervical |
Euthanized |
|
Table 1. Reported peripheral ganglioneuroblastomas in veterinary species. |
|||
The factors that lead to the persistence and subsequent neoplastic transformation of these progenitor cells are incompletely understood in both human and veterinary medicine.
In humans, most neuroblastic tumors carry an excellent prognosis if detected early in life; however, an unpredictable subset of human neuroblastic tumors carries increased risk and a poorer prognosis. The most well-characterized marker of these high-risk tumors is increased expression of MYCN, a transcription factor in the MYC family, which occurs in approximately 25% of pediatric neuroblastomas.6 Rodent studies have determined that MYCN, like MYC, can induce cellular proliferation and cell cycle progression in quiescent cells; however, there is a spatiotemporal difference between the two transcription factors. MYC is expressed in a broad spectrum of tissues and gradually subsides over time in adult mice.6 By contrast, MYCN is found only during early developmental stages and is expressed most substantially in the forebrain, hindbrain, and kidneys of newborn mice.6
Given its role in early development, it is perhaps unsurprising that the degree of differentiation in neuroblastoma cells is negatively correlated with MYCN expression, with more differentiated tumors characterized by lower levels of MYCN than less differentiated tumors.6 MYCN is also involved in maintaining the multipotency and capacity for self-renewal characteristic of the neural crest cells from which neuroblastic tumors arise, and mouse models have induced neuroblastomas by targeted overexpression of MYCN in migrating neural crest cells.6
MYCN’s contribution to the stem cell-like state of neural crest and neoplastic cells is multifactorial. MYCN increases expression of proliferation-permissive proteins such as CDK1 and CDK4, promotes angiogenesis by stimulating VEGF production, and facilitates cell survival through the p53-blocking protein MDM2.6 MYCN also inhibits the production of cell cycle arrest proteins and transcription factors that promote differentiation, down regulates immune surveillance by MCP-1, and creates a pro-metastasis environment through, among other actions, blocking production of E-cadherin and certain integrins.6
In dogs and humans, clinical signs vary widely depending on tumor localization and extent, and on the presence or absence of any paraneoplastic syndromes.3,14 In humans, 65% of peripheral neuroblastic tumors arise in the abdomen, with half of these originating in the adrenal medulla.14 With local or regional disease, patients may be asymptomatic, while more extensive disease may cause abdominal distention and pain, Horner’s syndrome (for cervical tumors), and episodic secretory diarrhea due to tumor production of vasoactive intestinal peptides.3,14
Due to the paucity of published case reports, the prognoses and biologic behaviors of canine peripheral neuroblastic tumors are hard to predict. In a recent case study of canine neuroblastomas, the majority of the dogs were either euthanized at the time of diagnosis or lost to follow up; of those submitted for necropsy, metastatic disease was discovered in 4/9 dogs, most commonly in the liver.3
This week’s moderator, Dr. Andrew Miller, Associate Professor and Section Chief of the Department of Population Medicine and Diagnostic Sciences at the Cornell University College of Veterinary Medicine, began discussion by reviewing the characteristics of various embryonal tumors. This heterogenous group of tumors includes central and peripheral neuroblastoma, ganglioneuroblastoma, medulloblastoma, and primitive neuroectodermal tumors, all of which can occur as paraspinal masses that compress the spinal cord. As a definitional matter, Dr. Miller noted that the term “primitive neuroectodermal tumor,” or PNET, is no longer used in human neuropathology due to the World Health Organization’s reclassification of nervous system tumors based on genetic and molecular markers. In veterinary medicine, the umbrella term “PNET” persists as the genetic mutations underlying nervous system tumors and the molecular methods used to diagnose them are less fully developed.
Ganglioneuroblastomas are generally rare in veterinary medicine, and diagnosis requires clear, obvious ganglion cell differentiation, neuroblasts, and some amount of Schwann cells and Schwannian stroma. Dr. Miller noted these elements in the examined slide and called attention to the large areas of confluent necrosis, an indication that the neoplasm was aggressive and rapidly growing.
Discussion of the morphologic diagnosis was blissfully straightforward, with only momentary debate about tissue localization. Conference participants were not able to detect the tissue’s mediastinal origin from the H&E section alone and thus chose to omit any reference to a specific anatomic location.
References:
- Alessi S, Grignani M, Carone L. Ganglioneuroblastoma: Case report and review of the literature. J Ultrasound. 2011; 14(2):84-88.
- Allen D, Swayne D, Belknap JK. Ganglioneuroma as a cause of small intestinal obstruction in the horse: a case report. Cornell Vet. 1989;79(2):133-141.
- Arenas-Gamboa AM, Tanabe M, Edwards J, Storts R. Peripheral neuroblastomas in dogs: a case series. J Comp Pathol. 2014; 150(4):361-365.
- Cole DE, Migaki G, Leipold HW. Colonic Ganglioneuromatosis in a Steer. Vet Path- ol. 1990;27(6):461-462.
- Goto M, Yonemaru K, Hirata A, Furuhashi H, Yanai T, Sakai H. Lingual ganglioneuroma in a dog. J Vet Med Sci. 2018; 80(3):488-491.
- Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harbor Perspect Med. 2013;3(10):a014415.
- Inoue R, et al. Cardiac ganglioneuroma in a juvenile pig. J Vet Med Sci. 2016;78(1): 117-119.
- Kobayashi R, Ohsaki Y, Yasuno K, et al. A malignant and metastasizing feline cardiac ganglioneuroma. J Vet Diagn Invest. 2012;24(2):412-417.
- Koullouros M, Candler S, Smith C, Olak-kengil S. Appendicitis and ganglioneuroma-an unusual co-existence. J Surg Case Rep. 2022;2022(1):rjab632.
- Nakamura K, Ochiai K, Kadosawa T, Kimura T, Umemura T. Canine ganglioneuroblastoma in the oral mucosa. J Comp Pathol. 2004;130(2):205-208.
- Omi K, Kitano Y, Agawa H, Kadota K. An immunohistochemical study of peripheral neuroblastoma, ganglioneuroblastoma, anaplastic ganglioglioma, schwannoma and neurofibroma in cattle. J Comp Pathol. 1994;111(1):1-14.
- Paris JK, McCandlish IAP, Schwarz T, Simpson JW, Smith SH. Small intestinal ganglioneuromatosis in a dog. J Comp Pathol. 2013;148(4):323–328.
- Park CH, Shiwa N, Kimitsuki K, Kakizaki T, Watanabe D. Cervical ganglioneuroblastoma in a new born Japanese Black calf. J Vet Med Sci. 2018;80(5):755-759.
- Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am. 2008;55(1):97-120.
- Pereira PR, Tagliari NJ, Leite-Filho RV, Schaefer G da C, Costa FVA da, Pavarini SP. Facial nerve ganglioneuroblastoma in a feline leukemia virus-positive cat. Ciênc Rural. 2017;47(5)1-5.
- Porter BF, Storts RW, Payne HR, Edwards JF. Colonic ganglioneuromatosis in a horse. Vet Pathol. 2007;44(2):207–210.
- Salvadori C, Cantile C, Massari F, Chiti L, Colombo S, Abramo F. Footpad peripheral ganglioneuroblastoma in a dog. Vet Dermatol. 2019;30(4):346-e100.
- Schulz KS, Steele KE, Saunders GK, Smith MM, Moon ML. Thoracic ganglioneuroblastoma in a dog. Vet Pathol. 1994;31(6):716–718.
- Shimada H, Chatten J, Newton WA, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73(2):405-416.
- Shimada H, Umehara S, Monobe Y, et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors. Cancer. 2001;92(9):2451-2461.
- Spugnini EP, Citro G, Dotsinsky I, Mudrov N, Mellone P, Baldi A. Ganglioneuroblastoma in a cat: a rare neoplasm treated with electrochemotherapy. Vet J Lond Engl 1997. 2008;178(2):291-293.
- Uchida K, Murakami T, Tometsuka T, Iwakiri A, Yamaguchi R, Tateyama S. Peripheral neuroblastoma and primitive neuroectodermal tumor in Japanese black cattle. J Vet Med Sci. 1998;60(7):871–875.
- Yener Z, Kiran MM. Undifferentiated Ganglioneuroblastoma in a Sheep. J Comp Pathol. 2002;126(2):216-219.