Signalment:
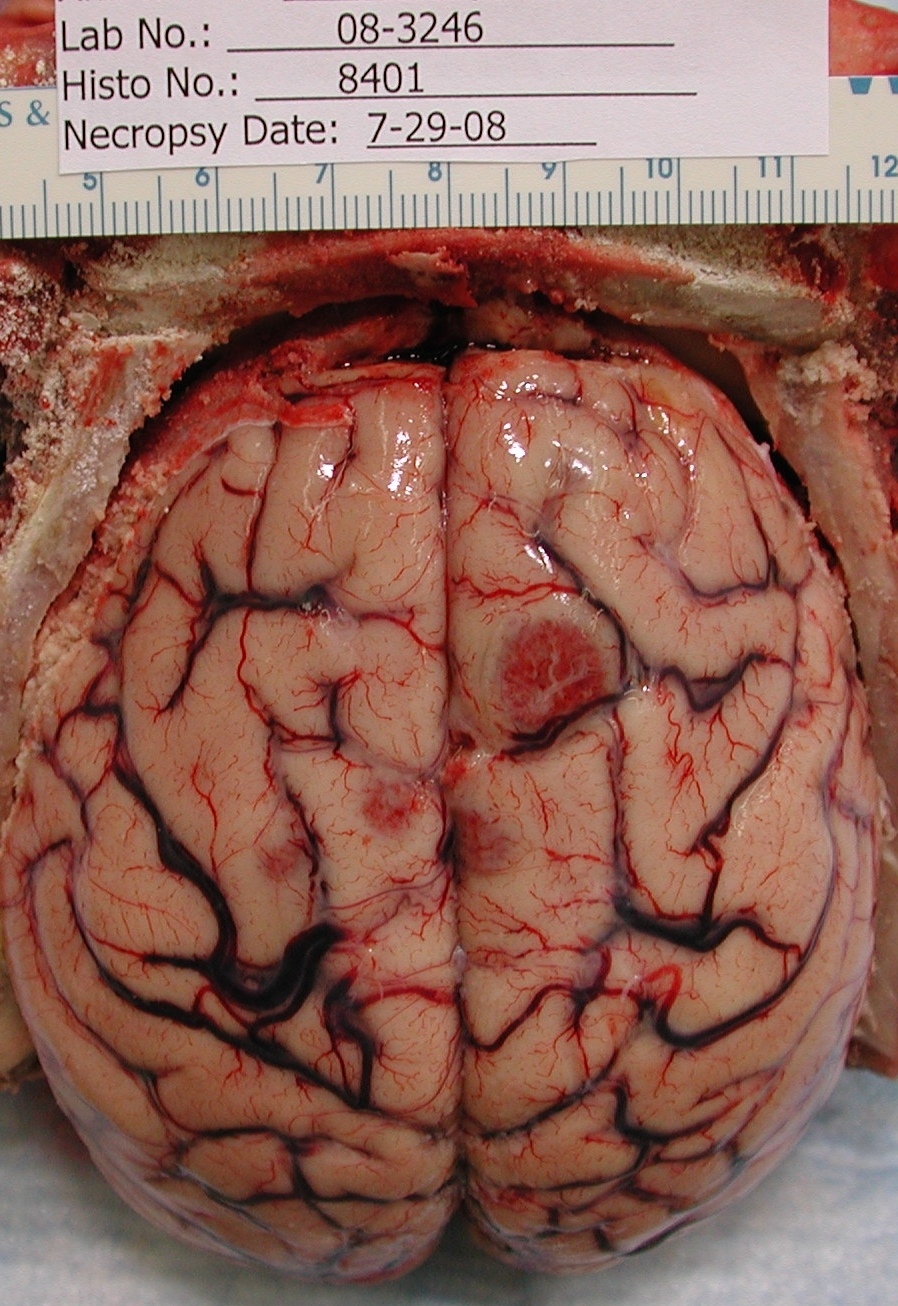
Gross Description:
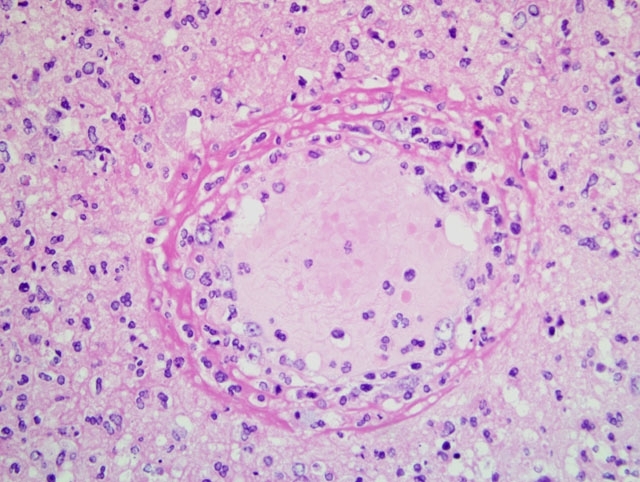
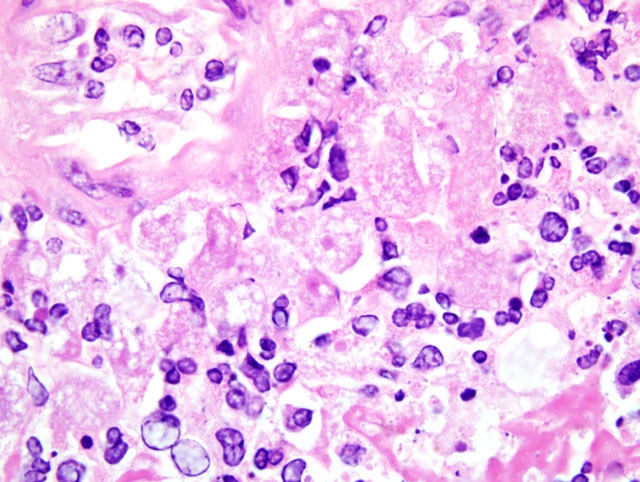
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Day 1: Leukocytosis (16.3 k/μl); neutrophilia (13.3 k/μl); and hypokalemia (2.6 mEq/L).
Day 3: Leukocytosis (35.5 k/μl); neutrophilia (31.2 k/μl); and hypokalemia (2.5 mEq/L).
Day 4: Leukocytosis (28.7 k/μl); neutrophilia (22.4 k/μl); and hypokalemia (2.8 mEq/L).
Day 5: Leukocytosis (28.0 k/μl); neutrophilia (22.2 k/μl); and hypokalemia (2.7 mEq/L).
Condition:
Contributor Comment:
JPC Diagnosis:
Conference Comment:
This case was reviewed in consultation with Dr. Christopher Gardiner, Consulting Parasitologist for the AFIPs Department of Veterinary Pathology; he noted that the large size of the Balamuthia mandrillaris trophozoites is helpful in distinguishing it from those of Naegleria fowleri and Acanthomoeba sp., the two other free-living amoebae most commonly implicated in human and animal disease. Only one case of amebic encephalitis has been attributed to a fourth free-living amoeba, Sappinia diploidea. During the conference, participants reviewed the freeliving amoebae, emphasizing the important distinguishing morphologic features. Acanthamoeba sp. and B. mandrillaris are closely related to one another phylogenetically, but are distant from N. fowleri and S. diploidea. While most B. mandrillaris trophozoites are uninucleate, binucleate forms may be seen, and the presence of multiple nucleoli may be useful in differentiating B. mandrillaris from Acanthamoeba sp. Although trophozoites are more numerous than cysts, the presence of cysts in brain tissue is useful in excluding N. fowleri, as only B. mandrillaris and Acanthamoeba sp. form cysts in the brain. However, the absence of cysts does not exclude a diagnosis of BAE, as cyst formation is not consistent, as the present case illustrates. When present, the cyst wall of B. mandrillaris is unique in that it is three-layered (i.e. composed of an outer ectocyst, inner endocyst, and intervening mesocyst) and lacks pores, whereas the cyst walls of Acanthamoeba sp. and N. fowleri are two-layered with pores.(3,4)
As mentioned by the contributor, differences in epidemiology and pathophysiology are also useful in differentiating the diseases caused by free-living amoebae. Acanthamoeba sp. generally only produces encephalitis in immunocompromised patients, whereas Balamuthia amebic encephalitis (BAE) is reported in both the immunocompetent and immunocompromised. By contrast, N. fowleri causes primary amebic meningoencephalitis (PAM) in immunocompetent children and young adults, classically within days of exposure to warm fresh waters. Primary amebic meningoencephalitis due to N. fowleri has also been reported naturally in bovids and a tapir (see WSC 2007-2008, Conference 22, case IV) and experimentally in a number of mammalian species. Of note, most humans with BAE present with characteristic skin lesions that predate central nervous system signs; such lesions are not characteristic of PAM produced by N. fowleri. Intriguingly, individuals of Hispanic origin are overrepresented among human BAE cases in the United States; unique environmental exposures and genetic predispositions have been proposed, but an explanation for this disparity has not been definitively proven. Among animals, BAE is most common in nonhuman primates, but has also been diagnosed in dogs, a sheep, and a horse.(3,4)
The major features that characterize and distinguish the pathogenic free-living amoebae are summarized in the following table, adapted from Schuster and Visvesvara:(3)
| Pathogenic Free-living Amoebae | |||
|---|---|---|---|
| Feature | Balamuthia mandrillaris | Acanthamoeba sp. | Naegleria fowleri |
| Diseases | Balamuthia amebic encephalitis (BAE); cutaneous and sinus infections | Amebic encephalitis; cutaneous and sinus infections* | Primary amebic meningoencephalitis (PAM) |
| Risk Factors | Immunocompromised status (also occurs in immuno-competent); breaks in skin contaminated with soil | Immunocompromised status | Activity in warm fresh waters; diving; not associated with immuno-compromised status |
| Incubation period | Weeks to years | Weeks to months | Days |
| Trophozoite stage | 12-60 um | 15-30 um | 15-30 um |
| Flagellate stage | Not found | Not found | Flagellate stage with 2 flagella |
| Cyst stage | 3-layered wall lacking pores; 10-30 um diameter; cysts form in brain tissue | 2-layered wall with pores; 10-15 um diameter; cysts form in brain tissue | 2-layered wall with pores; 7-15 um diameter; cysts do not form in brain tissue |
| *Acanthamoeba sp. also is associated with amebic keratitis in immunocompetent humans; risk factors include soft contact lens wear and wearing contact lenses while swimming; incubation period is days (versus weeks to months in systemic infections). | |||
References:
2. Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, Vugia D, Bakardjiev A, Azimi P, Maddux-Gonzalez M, Martinez AJ, Visvesvara GS: Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. Journal of Clin Microbiol 41:3175-3180, 2003
3. Schuster FL, Visvesvara GS: Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34:1001-1027, 2004
4. Siddiqui R, Khan NA: Balamuthia amoebic encephalitis: an emerging disease with fatal consequences. Microb Pathog 44:89-97, 2008
5. Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M: Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol 28:2750-2756, 1990
6. Visvesvara GS, Moura H, Schuster FL: Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1-26, 2007