Signalment:
13-year-old Bay Warmblood mare (
Equus caballus).Since December 2012 the horse has had fever and increased respiratory rate. The horse started being treated by Baytril and Naxcel, but did not improve. At this time, the animal was presented to Tufts University Cummings School of Veterinary Medicine. Transtracheal aspirate (TTA) fluid was cultured, but no pathogen was found. Thoracic radiographs show that the lung has a diffuse, severe, interstitial, and nodular pattern. The TTA fluid was positive for equine herpesvirus 5 by PCR test. The biopsy of lung tissue reveals fibrotic changes. Later, the mare developed laminitis and was humanely euthanized.
Gross Description:
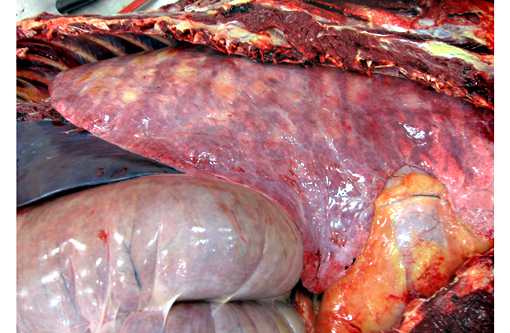
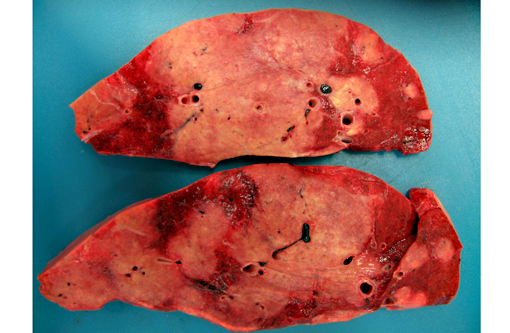
All lung lobes contain multifocal to coalescing, firm, white to tan, variably-sized, slightly-raised nodules, ranging from 0.5 cm x 0.3 cm x 15 cm to 15 cm x 9 cm x 7 cm. The cut surface of the nodules is tan and firm. Lung parenchyma between the nodules is dark red and markedly congested. The tracheobrochial lymph nodes are enlarged ranging from 5 cm x 3 cm x 2.5cm to 9 cm x 4 cm x 3 cm.
The midsagittal section of hoof of both forelimbs revealed the dorsal surface of third phalanx is widely separated (up to 0.5 cm) from the epidermal laminae of the inner surface of hoof wall with ventral rotation of the tip of the third phalanx. The space between them is filled with tan-white firm tissue.
Histopathologic Description:
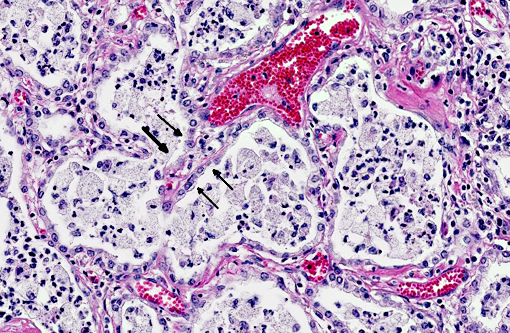
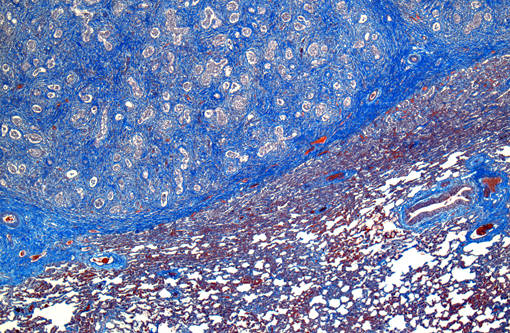
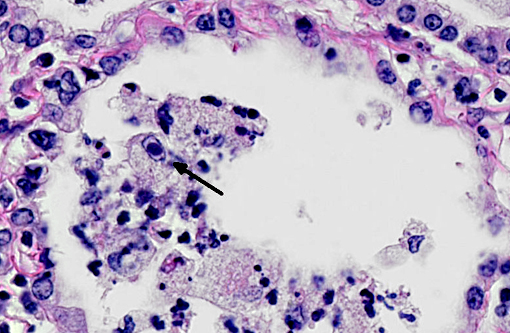
In a multifocal to regionally extensive area, the alveolar septa are variably thickened up to 3 times by fibrous connective tissue composed of well differentiated to plump fibroblasts admixed with moderate to high numbers of foamy macrophages, neutrophils, lymphocytes, plasma cells, and necrotic cellular and karyorrhectic debris. The alveoli are lined by plump cuboidal cells (type II pneumocyte hyperplasia) and filled with moderate to marked numbers of giant foamy macrophages, intact and degenerate neutrophils, and necrotic epithelial cell debris with karyorrhexis and karyolysis. Occasionally, macrophages contain up to 5 μm diameter eosinophilic to amphophilic intranuclear inclusion bodies that marginate chromatin. Bronchi and bronchioles within the affected areas contain a minimal to mild amount of cellular and karyorrhectic debris sometimes with few degenerate neutrophils. Occasionally, bronchi have mild epithelial hyperplasia. Multifocally, there is moderate perivascular, peribronchial and peribronchiolar fibrosis admixed with moderate number of macrophages, lymphocytes, plasma cells, and few neutrophils. Multifocally, the adjacent relatively normal lung parenchyma has mild amounts of eosinophilic, homogenous, acellular material in the alveoli and the interstitia is diffusely thickened by increased clear spaces (edema).
Morphologic Diagnosis:
Multifocal to regionally extensive moderate pulmonary fibrosis and bronchointerstital pneumonia with intrahistiocytic intranuclear viral inclusion bodies consistent with equine multinodular pulmonary fibrosis.
Lab Results:
Transtracheal aspirate (TTA)
1. PCR test: Equine herpesvirus 5 positive
2. Bacterial culture: Negative
Condition:
Equine Multinodular Pulmonary Fibrosis (EMPF)
Contributor Comment:
Equine multinodular pulmonary fibrosis (EMPF) is a recently reported fibrotic interstitial lung disease in adult horses.(5) No breed or sex predilection was determined. The mean age of affected horses was 13 years. The common clinical presentations are weight loss, pyrexia, and tachypnea.(5,6) Thoracic radiographs often demonstrate an interstitial to nodular pulmonary pattern. Grossly, there are two major manifestations. The more common lesions are numerous pale tan-white, coalescing, firm nodules, involving all lung lobes. Sometimes, the lesions form masses, which makes it difficult in differentiating from neoplasia. The borders are discrete where they meet unaffected lung. The less common one often only demonstrates multiple discrete nodules separated by grossly normal lung.(5) Histopathologically, there is often marked multifocal and regionally extensive interstitial and alveolar septa fibrosis with often preservation of cuboidal epithelial cells (type II pneumocytes) forming alveolar-like structures. The lumen of the structures is often filled with mild to moderate number of intact and degenerate neutrophils, macrophages and cellular and karyorrhectic debris. Dark eosinophilic intranuclear viral inclusion bodies may be seen in the foamy macrophages.(4-6)
Equine herpesvirus 5 (EHV-5), a gamma herpesvirus, is consistently detected in the lung tissue of affected horses.(1,4-6) And, compared to other tissues, lung has a remarkably higher viral load than in other organs, especially within the pulmonary fibrotic lesions. These evidences support the high correlation between EHV-5 and EMPF.(4) Interestingly, equine herpesvirus 2 and asinine herpesvirus 5 (AHV5), another two types of gammaherpes viruses, may also be detected in the lesions.(1,4) Although these gammaherpes viruses are detected in the lesions, the pathogenic roles of them are currently still unclear.
JPC Diagnosis:
Lung: Pneumonia, interstitial, necrotizing and fibrosing, focally extensive, severe, with marked type II pneumocyte hyperplasia, neutrophilic and histiocytic alveolitis, and rare intrahistiocytic viral intranuclear inclusion bodies.
Conference Comment:
Conference participants observed that pleural arteries are often quite prominent, with hypertrophied smooth muscle within the tunica media and abundant collagen; this is likely an indication of severe pulmonary hypertension secondary to diffuse fibrosis. The differential diagnosis for the gross and histopathological lesions in this case includes silicate pneumoconiosis, which generally exhibits variable amounts of granulomatous inflammation in association with pulmonary interstitial fibrosis;(5) idiopathic pulmonary fibrosis, which more commonly affects foals than adults and is attributed to diffuse alveolar damage;(1,5) paraquat/diquat toxicosis, which, although rare, also causes fulminant pulmonary fibrosis;(2) and exercise-induced pulmonary hemorrhage, which has large areas of pulmonary fibrosis admixed with numerous hemosiderophages.(2) Participants also noted that the fibrosis in this case did not appear as nodular as normally described in cases of EMPF; this led to the suggestion of equine herpes virus (EHV)-1, which is most notorious as a cause of abortion in horses, but may also cause respiratory disease and encephalomyelitis, or EHV-4 (equine rhinopneumonitis virus) as possible etiologies.(3)
In conference, the moderator led a detailed review of the immune response as it relates to this case, touching upon various general pathology concepts such as the components of innate immunity (see
WSC 2013-2014 conference 3, case 1 for an abridged examination of toll-like receptors), the cellular and humoral arms of the adaptive immune response, the MHC molecule, and potential paths of differentiation for T-helper cells (see
WSC 2013-2014 conference 2, case 2 for a review of TH1 versus TH2 reactions). Additionally, participants briefly discussed an interstitial pneumonia of donkeys which has been reported in association with asinine herpesvirus. This disease differs from EMPF in that it is a diffuse inflammatory disease with syncytial cell formation without viral inclusions; interstitial fibrosis is considered a secondary component.(5)
References:
1. Back H, Kendall A, Grand³n R, et al. Equine multinodular pulmonary fibrosis in association with asinine herpesvirus type 5 and equine herpesvirus type 5: a case report. Acta Vet Scand. 2012;54:57.
2. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Elsevier; 2007:549, 574-575.
3. MacLachlan NJ, Dubovi EJ. Fenners Veterinary Virology. 4th ed. London, UK: Academic Press; 2011:188-190.
4. Marenzoni ML, Passamonti F, Lepri E, et al. Quantification of Equid herpesvirus 5 DNA in clinical and necropsy specimens collected from a horse with equine multinodular pulmonary fibrosis. J Vet Diagn Invest. 2011;23(4):802-806.
5. Williams KJ, Maes R, Del Piero F, et al. Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus- associated fibrotic lung disease. Vet Pathol. 2007;44:849862.
6. Wong DM, Belgrave RL, Williams KJ, et al. Multinodular pulmonary fibrosis in five horses. J Am Vet Med Assoc. 2008;232:898905.