Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 14
January 10th, 2018
CASE IV: 2 (white colored slide) (JPC 4065579).
Signalment: 1.5-year-old, female, Cavia porcellus, guinea pig.
History: The animal was a pet that developed severe abdominal enlargement. At surgery one of the ovaries was massively enlarged (approximately 6X4 cm).
Gross Pathology: None provided.
Laboratory results: None provided.
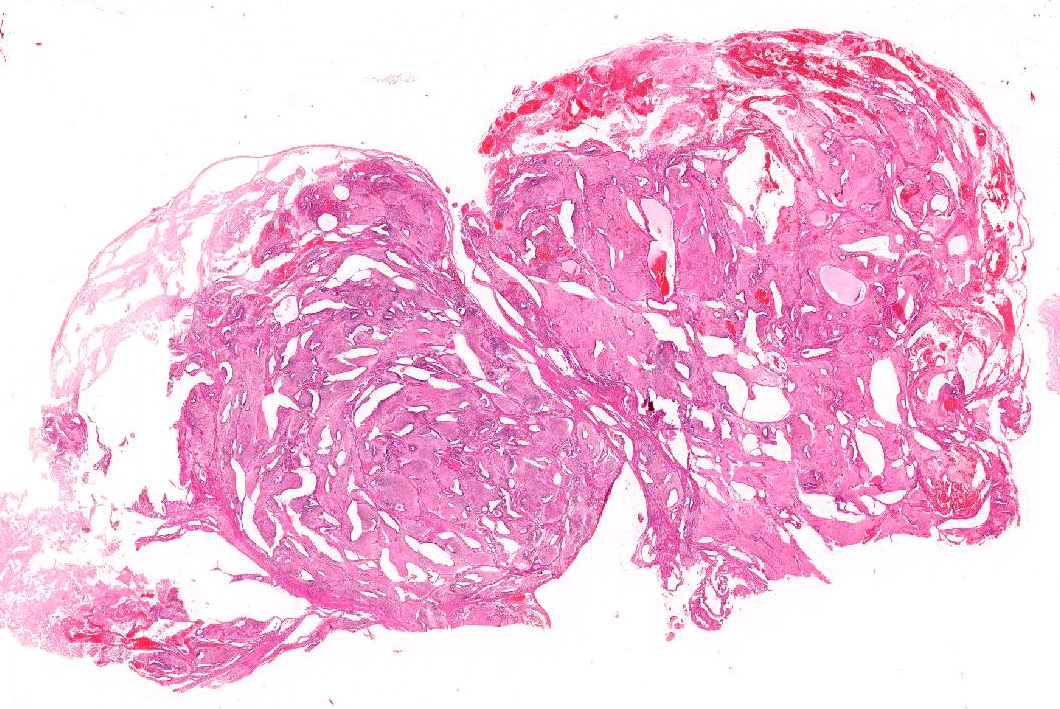
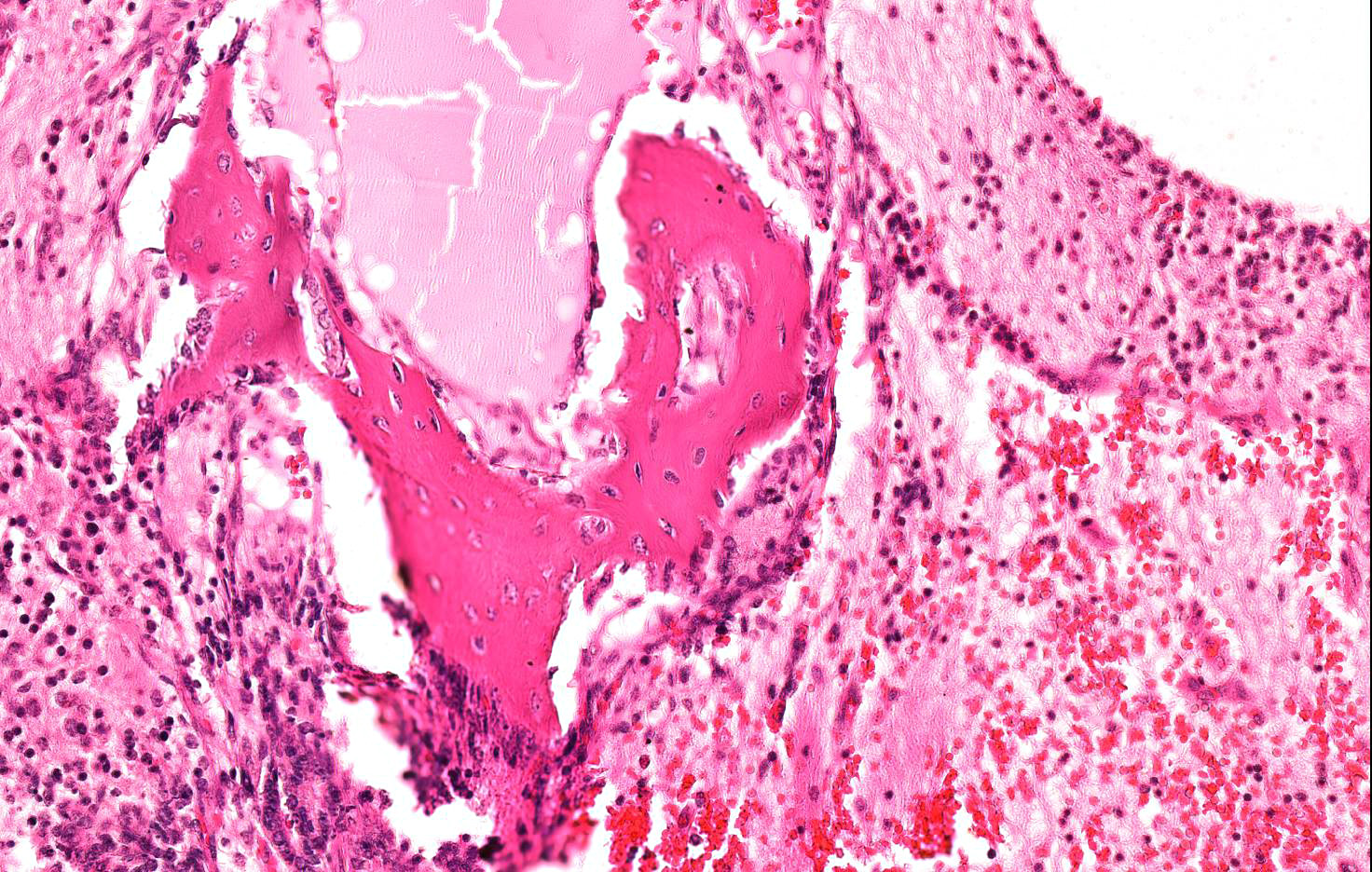
Microscopic Description: Almost 100% of the ovary (part of the tumor submitted) is replaced by an irregularly round, unencapsulated, poorly demarcated, densely cellular, pleomorphic, expansile neoplasm that extends to the cut borders of the sample provided. (The lesion was delimited by the ovarian capsule).
Neoplasm is composed of variably arranged cells with a derivation from both mature and embryonal elements of all three primordial germ cell layers, embedded in a variable amount of fibrovascular stroma.
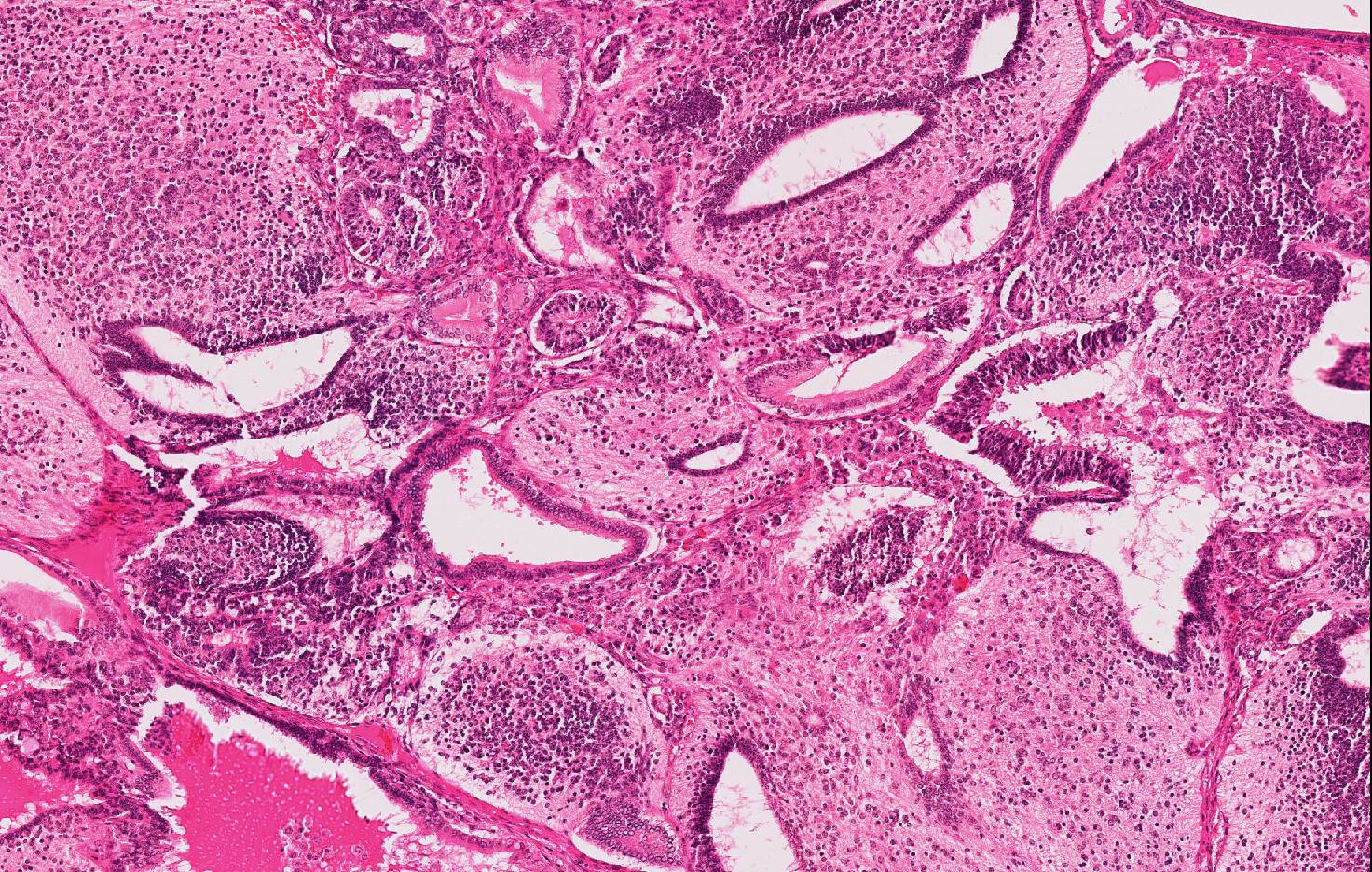
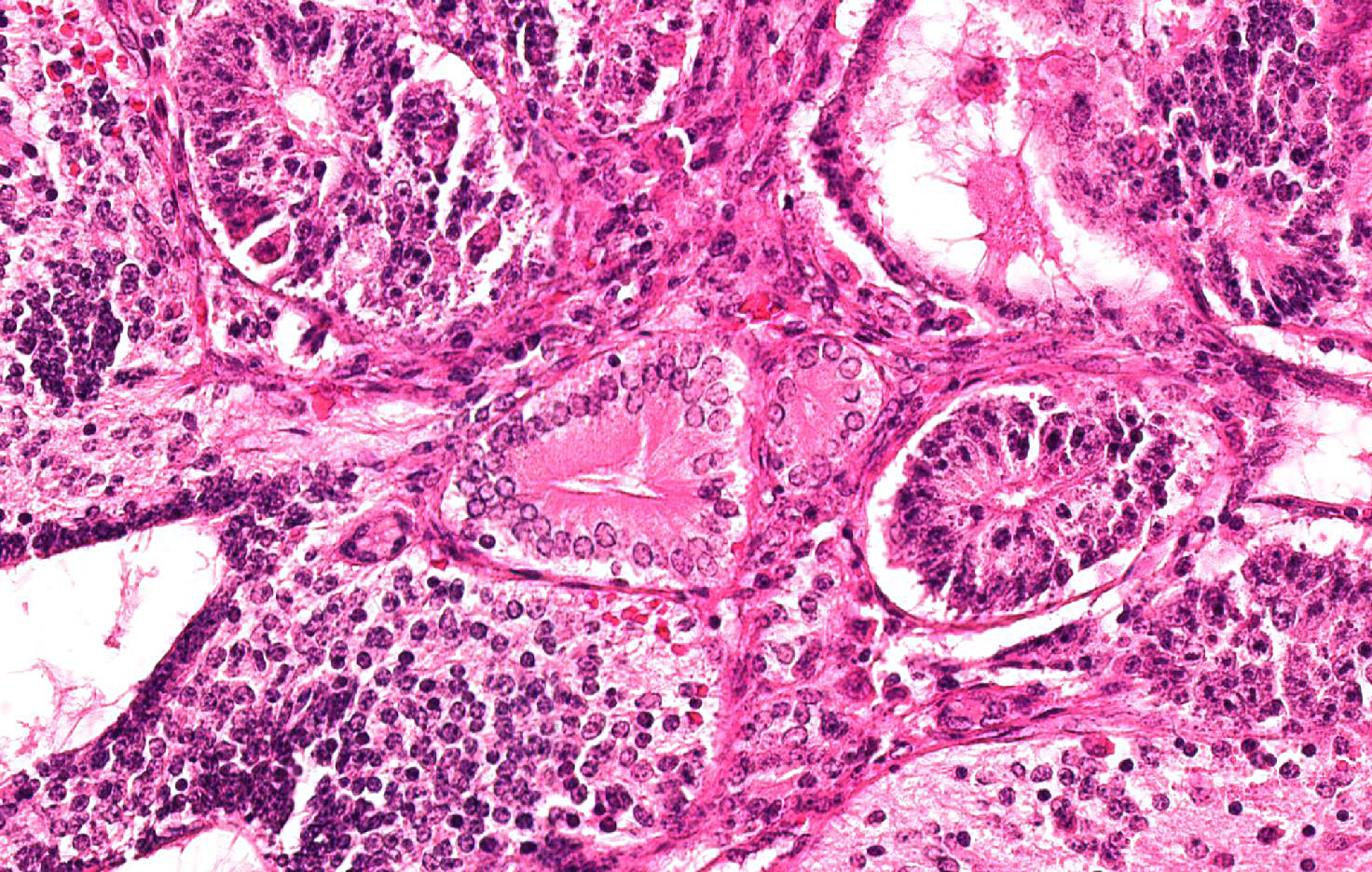
Approximately 60% of the neoplasm consists of neuroectodermal neoplastic cells that multifocally palisade around either central areas composed of brightly eosinophilic, homogeneous protein-rich material (neuroepithelial rosettes mimicking ventricular spaces and ependyma) surrounded by sheets of glial cells (mimicking glial production zone) or around blood vessels (pseudo-rosettes). Neoplastic cells are oval to cylindrical, 15-20 micron in diameter, have indistinct borders, high N/C ratio and central or palisading hyperchromatic oval nuclei with indistinct nucleoli. There are scattered larger (30-40 micron in diameter), polygonal to stellate cells with abundant, pale basophilic, granular cytoplasm and a central round nucleus with single prominent nucleolus (well differentiated neurons) embedded in an abundant, pale eosinophilic, vaguely fibrillar to vacuolated background substance (neuropil).
Ectoderm is represented by few tubules and cystic spaces lined by single or multiple layers of cuboidal to columnar, 20-24 micron in diameter, epithelial cells with distinct cell borders, polarized nuclei and evident apical capitation of the cytoplasm (consistent with apocrine gland origin).
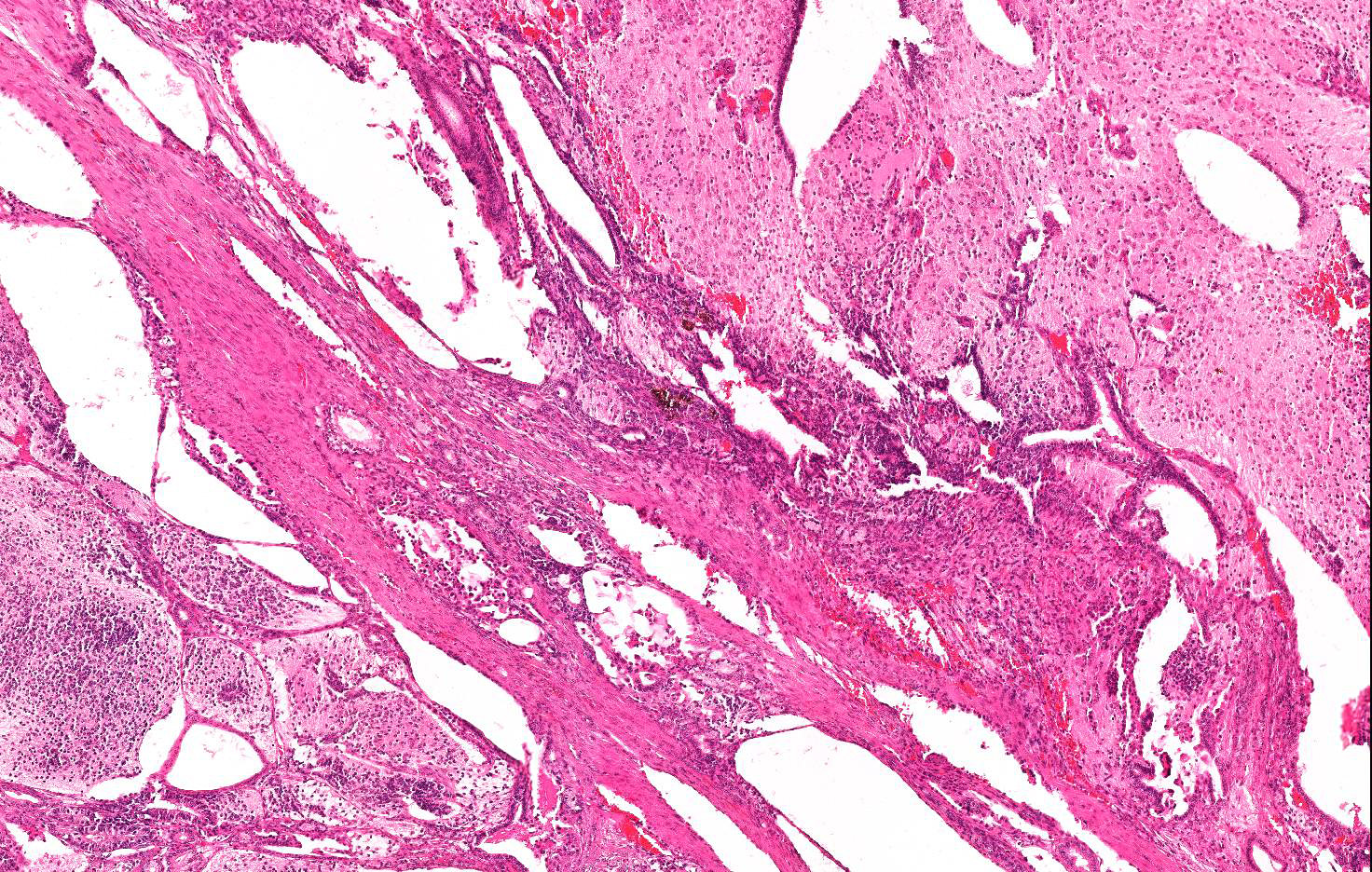
Mesodermal elements include bundles of fusiform cells 30-35 micron in length with indistinct cell borders, abundant pale eosinophilic cytoplasm and central, cigar shaped nuclei (well differentiated smooth muscle tissue), multiple focal islands of oval to stellate cells loosely embedded in abundant pale basophilic, myxoid matrix (consistent with myxoid to embryonal cartilage- not included in all sections) and rare, small multifocal areas of mineralized lamellar bone with intralacunar round to oval osteocytes (not included in all sections).
Endoderm comprises multiple, variably sized, cystic spaced, up to 0,5 mm in diameter, filled with variable amounts of amphophilic, fibrillar to granular material (mucus), lined by pseudostratified epithelium composed of columnar to bottle-shaped cells, with basal to centrally located nucleus and apical ciliated border (consisting of respiratory epithelium) or few acini and tubules of epithelial polygonal cells with pale eosinophilic cytoplasm expanded by secretory pale vacuoles and flattened basal nuclei (consisting with salivary or goblet cells).
Cellular atypia such as anisocytosis anisokaryosis are mild in all cell populations; occasional multinucleated, undifferentiated neoplastic elements are seen; mitoses are fewer than 1 per 10 HPF.
Almost 25% of the mass is composed of elevated numbers of extravasated erythrocytes (hemorrhages) admixed with a meshwork of fibrillar lightly eosinophilic extracellular material (fibrin) and foci of pale eosinophilic, granular to amorphous extracellular material mixed to karyorrhectic cell debris (liquefactive necrosis).
Contributor’s Morphologic Diagnosis: Ovary, ovarian teratoma, guinea pig (Cavia porcellus)
Contributor’s Comment: Teratomas are complex neoplasms composed of tissues representative of at least two, (sometimes all three) primordial germinal layers that are the ectoderm (nervous tissue skin including adnexa), mesoderm (connective tissue, muscle, bone, cartilage, and urogenital and cardiovascular system), and endoderm (gastrointestinal and respiratory epithelium, including their glandular structures).9 Teratomas are tumors considered to emerge from proliferation of totipotent stem cells occurring physiologically in gonads and that sometimes may reside in abnormal location due to anomalous migration or lack of regression of midline embryonic rest. Such cells have the capacity to differentiate into any of the cells types of the adult body.6 Teratomas usually occur in the gonads9,16 and more frequently in the ovary where they may cause spherical to ovoid severe enlargement with a gross aspect of solid and cystic areas on cut surface9 but reports about extragonadal teratomas, involving cutaneous structures, the alimentary tract, the kidneys, and retroperitoneal and retrobulbar spaces, as well as other systems, also exist.
Teratomas are considered uncommon in domestic animals9,16 and have been reported in humans, nonhuman primates, dog, cat, horse (base of the ear), sheep, ox, rabbit, swine, laboratory rodents, ferret (adrenal gland), poultry, a blue heron, frogs, hares, squirrel, a spot-necked otter, a woodchuck, turtles, porpoise, hedgehog, red-eared slider and a giraffe.4,10,11,12,15,17,20 In guinea pigs, primary tumors of the reproductive tract represent approximately 25% of spontaneous tumors.13 Among these, teratoma is the most frequent.19,21 Differential diagnosis in guinea pigs include cystic rete ovarii seen commonly in older sows.13 Teratomas in mice have been rarely reported in B6C3F1, LT/Sv, CD1, C3H strains, with the exception of inbred strain 129 mice. Indeed, approximately 1/3 of 129 teratoma substrain male mice develop spontaneous testicular teratomas.14 An ovarian teratoma with associated trisomy of chromosome 16 has been reported in a baboon.10
Classification of a teratoma as benign or malignant is based largely on the relative amount of primitive, undifferentiated cell content and type of tissues within the tumor: when all the components are well differentiated they are classified as benign (mature) teratomas; on the contrary, if undifferentiated cells/tissue are predominant, and immature neuro-ectodermal elements are over-represented, the tumor is termed teratocarcinoma and is considered malignant.6 In general, the presence of undifferentiated cells aggravates the prognosis, and teratomas with incompletely differentiated tissues should be considered potentially malignant.7,16
However, in the present case, despite the major composition by variably differentiated neuro-ectodermal tissue and the complete substitution of the ovarian parenchyma, a final diagnosis of teratoma and not of teratocarcinoma was made, due to the presence of several well differentiated and recognizable cell lines and absence of neoplastic emboli or distant metastasis. In animals most ovarian and prepuberal testicular teratomas are considered benign, whereas most post pubertal testicular tumors in men are malignant, suggesting a differential origin from benign and malignant cells respectively.8 The difference may reside in the human tolerance for parthenogenetic development of immature somatic ovarian cells into three germ layers while suppressing neoplastic cells, in contrast to the human male that differentiates malignant immature somatic cells less efficiently in the embryo.18 The k-FGF gene, a member of the family of fibroblast growth factor genes, is considered as a marker for murine malignant, testicular, teratoma.5
JPC Diagnosis: Ovary: Teratoma, Cavia porcellus, guinea pig.
Conference Comment: As an excellent review of teratomas is provided by the contributor, our conference comment will focus on ovarian tumors in general.
Ovarian tumors are classified based on embryologic origin: (1) epithelial (surface epithelium of modified mesoderm, rete ovarii, or subsurface epithelial structures (SES, in bitches); (2) germ cells; (3) ovarian stroma (sex cord stromal or gonadostromal). More specifically, sex cord stromal elements are the theca and granulosa cells and their luteinized derivatives which are essential to the formation of primary follicles.1 A review of ovarian follicle progression is prudent at this juncture. Primordial follicles (the most immature) are plentiful in the adult ovary located deep to the tunica albuginea and composed of a central oocyte surrounded by a layer of simple squamous follicle cells. Hormonal stimulation induces maturation to a primary follicle composed of the central oocyte which is developing, surrounded by a layer of cuboidal cells which proliferate to form a multilaminar (late primary) follicle. An antrum progressively forms as the follicle cells conjoin and leave an open fluid filled space as the zona pellucida forms around the outside of the follicle. The follicle is termed “secondary” once the antrum has widened into a C-shape. At this point, the follicle cells are called membrana granulosa and a layer of stromal cells form around the outer zona pellucida called the theca folliculi. The theca folliculi has two layers: the interna (a more cellular, inner vascular layer) and the externa (an outer layer of connective tissue). Finally, continued stimulation and growth leads to Graafian (tertiary) follicle formation in which the oocyte is surrounded by several layers of membrana granulosa cells auspiciously termed the cumulus oophorus. Following ovulation, granulosa and theca interna cells multiply, hypertrophy, and differentiate into granulosa and theca lutein cells which form the corpus luteum and produce progesterone. Assuming there is no pregnancy, the corpus luteum regresses during the end of diestrus to form a corpus albicans followed by atresia and resorption of the remaining structure.2
Ovarian epithelial tumors are most common in the bitch, arising from the SES, and tend to form cystic nodules that elevate the surface of the ovary. Metastasis of carcinomas occurs via implantation with ascites. Sex cord stromal tumors, on the other hand, are more common in the mare, cow, and queen and are named according to the originating cell type of the ovarian endocrine scheme (granulosa cell tumor, granulosa-theca cell tumor, thecoma, luteoma, Sertoli cell tumor of the ovary or lipid cell tumor). These tumors, being endocrine in origin, frequently produce hormones such as progesterone, estrogen, or inhibin resulting in systemic effects like anestrus, persistent estrus (nymphomania), masculinization, and blood dyscrasias. Granulosa cell tumors are the most common sex cord stromal tumor and are composed of aggregates of granulosa cells with the vague appearance of follicle formation surrounded and separated by a supporting stroma of spindle cells. Some tumors (more frequent in horses) contain Cal-Exner bodies, homogenous eosinophilic deposits within the center of the follicular structures, which are a useful microscopic feature. In dogs, granulosa cell tumors may appear to be composed of Sertoli cells. For that reason, this type is called Sertoli cell tumor of the ovary. Finally, germ cell tumors of the ovary can be broken down into two main categories: dysgerminoma or teratoma. Dysgerminomas are extremely rare in domestic animals and are composed of broad sheets of large cells with prominent nuclei and scant cytoplasm. Grossly, they are large, grey to white, and firm. Teratomas (described in detail above) are, by definition, composed of two or more germinal layers.1 The germ cell layers are ectoderm, mesoderm, and endoderm and form the components listed below.
Table 1: Germ cell layer derivatives3
|
Ectoderm |
Mesoderm |
Endoderm |
|
Epidermis of skin and its derivatives (sweat glands, hair follicles) |
Notochord |
Epithelial lining of digestive tract |
|
Epithelial lining of mouth and anus |
Skeletal system |
Epithelial lining of respiratory system |
|
Cornea and lens of eye |
Muscular system |
Lining of urethra, urinary bladder, and reproductive system |
|
Nervous system |
Muscular layer of stomach and intestine |
Liver |
|
Sensory receptors in epidermis |
Excretory system |
Pancreas |
|
Adrenal medulla |
Circulatory and lymphatic systems |
Thymus |
|
Tooth enamel |
Reproductive system (except germ cells) |
Thyroid and parathyroid glands |
|
Epithelium of pineal and pituitary glands |
Dermis of skin |
|
|
|
Adrenal cortex |
|
Conference attendees discussed the potential locations for this tumor because there was no apparent normal tissue present. One participant pointed out that even within completely effaced ovaries, there is at usually SES and surface epithelium remaining. It is unclear whether SES are present in guinea pig ovaries since available resources only confirmed their presence in canine ovaries.2,13 However, in some sections there were remnants of surface epithelium suggesting the submitted slide was truly ovarian in nature.
Contributing Institution:
DIVET-University of Milano
Via Celoria 10
20133 Milano Italy
http://eng.divet.unimi.it/ecm/home
References:
- Agnew DW, MacLachlan NJ. Tumors of the genital systems. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th Ames, IA: John Wiley & Sons, Inc.; 2017:690-698.
- Bacha WJ, Bacha LM. Female reproductive system. In: Color Atlas of Veterinary Histology. 2nd Ames, IA: Blackwell; 2006:221-223.
- Banks WJ. Epithelia. In: Reinhardt RW, Steube M, eds. Applied Veterinary Histology. 3rd St. Louis, MO: Mosby; 1993:48.
- Barlow AM, Couper D. Cutaneous teratoma in a wild roe deer. Vet Rec. 2006;159:211-212.
- De Anta JM, Monzó M, Peris B, Ruano. k-FGF protooncogene expression is associated with murine testicular teratogenesis but is not involved during mouse testicular development. Histol Histopathol. 1997;12:33-41.
- Ellenson LH, Pirog EC. The female genital tract. In: Robbins and Cotran, eds. Pathologic Basis of Disease. 8th ed., Philadelphia, PA: Saunders Elsevier; 2010:1047-1048.
- Klein MK. Tumors of the female reproductive system. Germ cell tumors. In: Withrow SJ, Vail DM, Withrow & MacEwen’s Small Animal Clinical Oncology. 4th St. Louis, MO: Saunders Elsevier; 2007: 610-611.
- Lakhoo K. Neonatal teratomas. Early Hum Develop. 2010; 86:643-647.
- MacLachlan NJ, Kennedy PC. Tumors of the genital systems. In: Meuten DJ, ed. Tumors in Domestic Animals. 3rd Ames, IA: Iowa State Press; 2002:554-555, 565-567.
- Moore CM, McKeand J, Witte SM, e al. Teratoma with trisomy 16 in a baboon (Papio hamadryas). Am J Primatol. 1998; 46:323-32.
- Murai A, Yanai T, Kato M, et al. Teratoma of the umbilical cord in a giraffe (Giraffa camelopardalis reticulata). Vet Pathol. 2007; 44:204-6.
- Newman SJ, Brown CJ, Patnaik AK. Malignant ovarian teratoma in a red-eared slider (Trachemys scripta elegans). J Vet Diagn Invest. 2003; 15:77-81.
- Percy DH, Barthold SW. Guinea Pig. In: Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 3rd Oxford, UK: Blackwell Publishing; 2007:248-249.
- Percy DH, Barthold SW. Mouse In: Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 3rd Oxford, UK: Blackwell Publishing; 2007:121.
- Schelling SH, Morton D. An extragonadal teratoma in a female cynomolgus monkey (Macaca fascicularis). J Med Primatol. 2015;44:113-5.
- Schlafer DH, Miller RB. Female genital system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th ed., Vol 3, Philadelphia, PA: Saunders Elsevier; 2007:450, 453-454.
- Shelling SH. Retrobulbar teratoma in a great blue heron. J Vet Diagn Invest. 1994; 6:514-516.
- Ulbright TM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Modern Path. 2005;18:S61-79.
- Vink HH. Ovarian teratomas in guinea-pigs: a report of ten cases. J Pathol. 1970;102:180-182.
- Williams BH, Yantis LD, Craig SL et al. Adrenal teratoma in four domestic ferrets (Mustela putorio furo). Path. 2001, 38:328-331.
- Willis RA. Ovarian teratomas in guinea-pigs. J Pathol Bacteriol. 1962; 84:237-239.