Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 11
December 6th, 2017
CASE III: 17-1539 (JPC 4102429).
Signalment: Adult,male, Stenella coeruleoalba, dolphin.
History: An adult male dolphin (Stenella coeruleoalba) was found dead on the beach with multiple signs of trauma and sent to Oregon State University for necropsy.
Gross Pathology: The animal was in good body condition and had numerous full thickness lacerations throughout the skin. Adjacent to both testes, there was a 3cm diameter abscess. The animal’s rectum contained dozens of well circumscribed, firm parasitic nodules.
Laboratory results:
Brucella ceti was isolated from brain tissue.
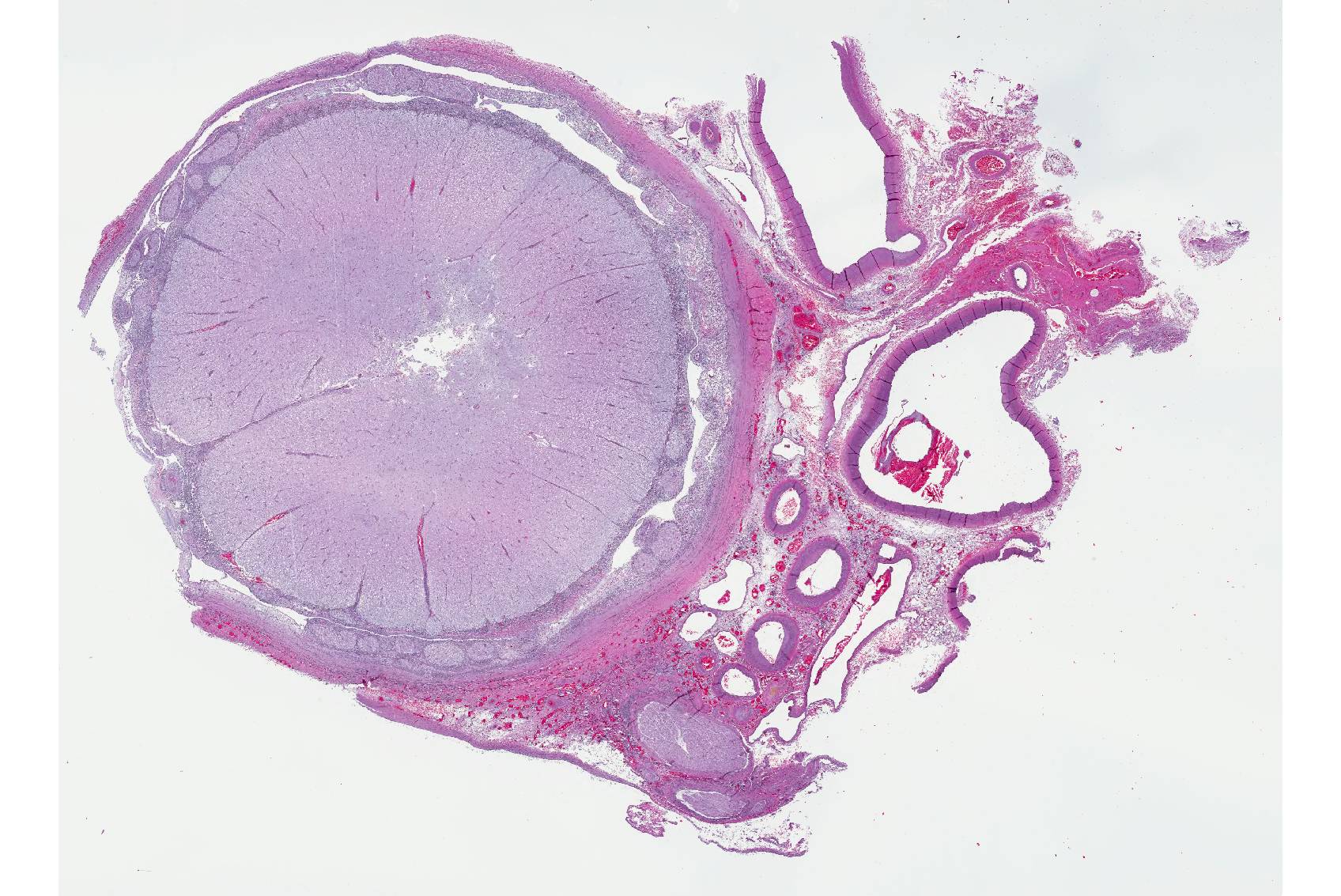
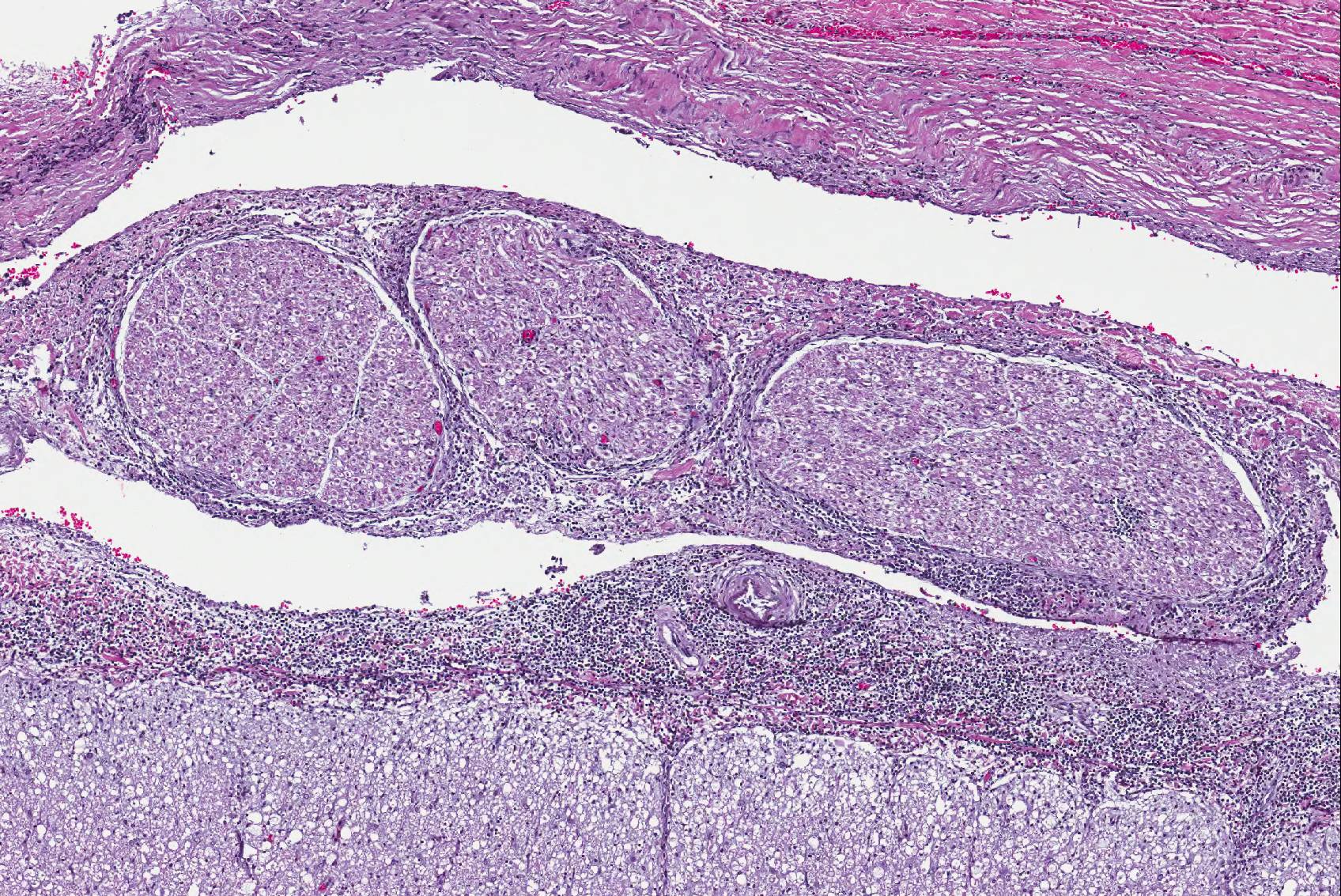
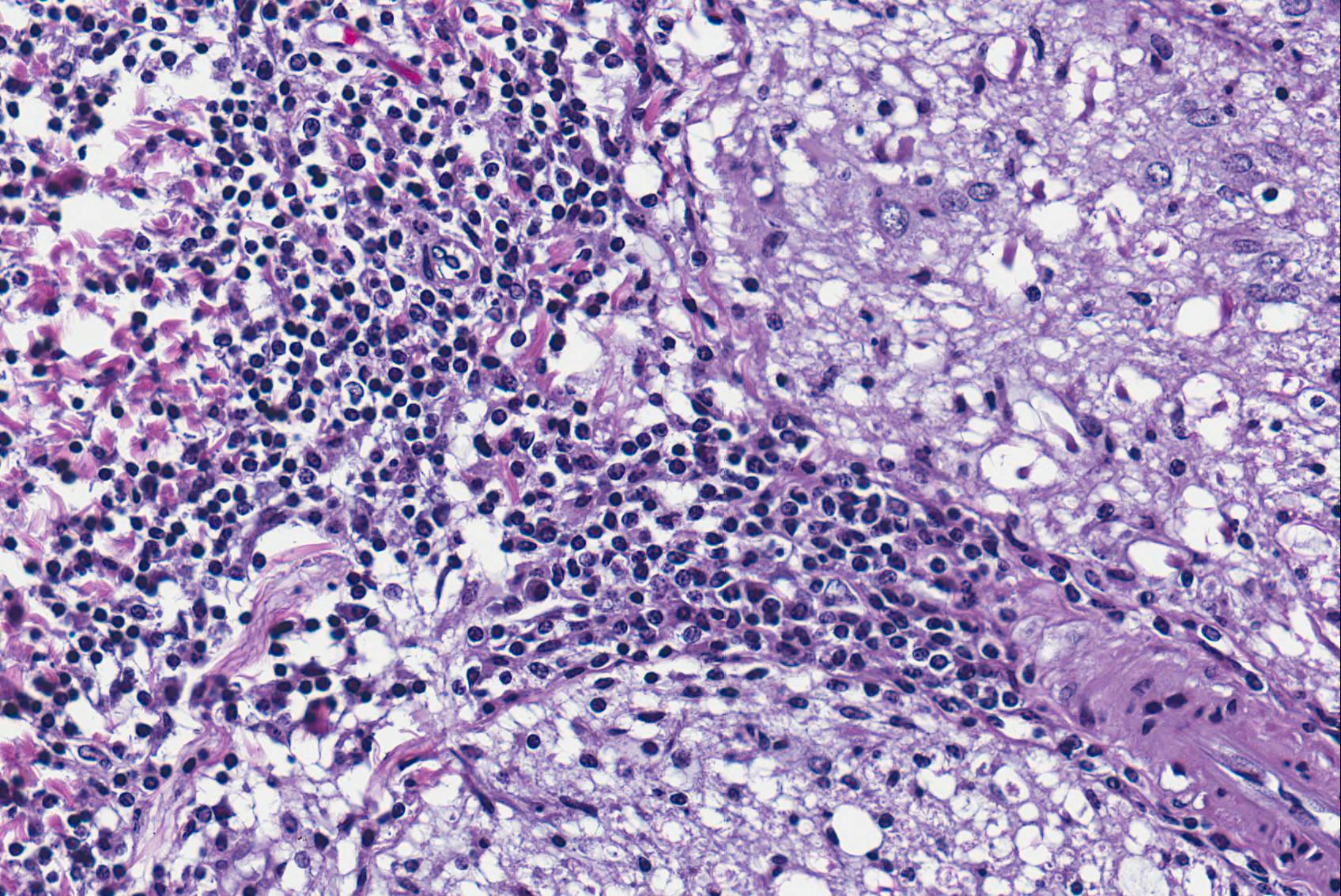
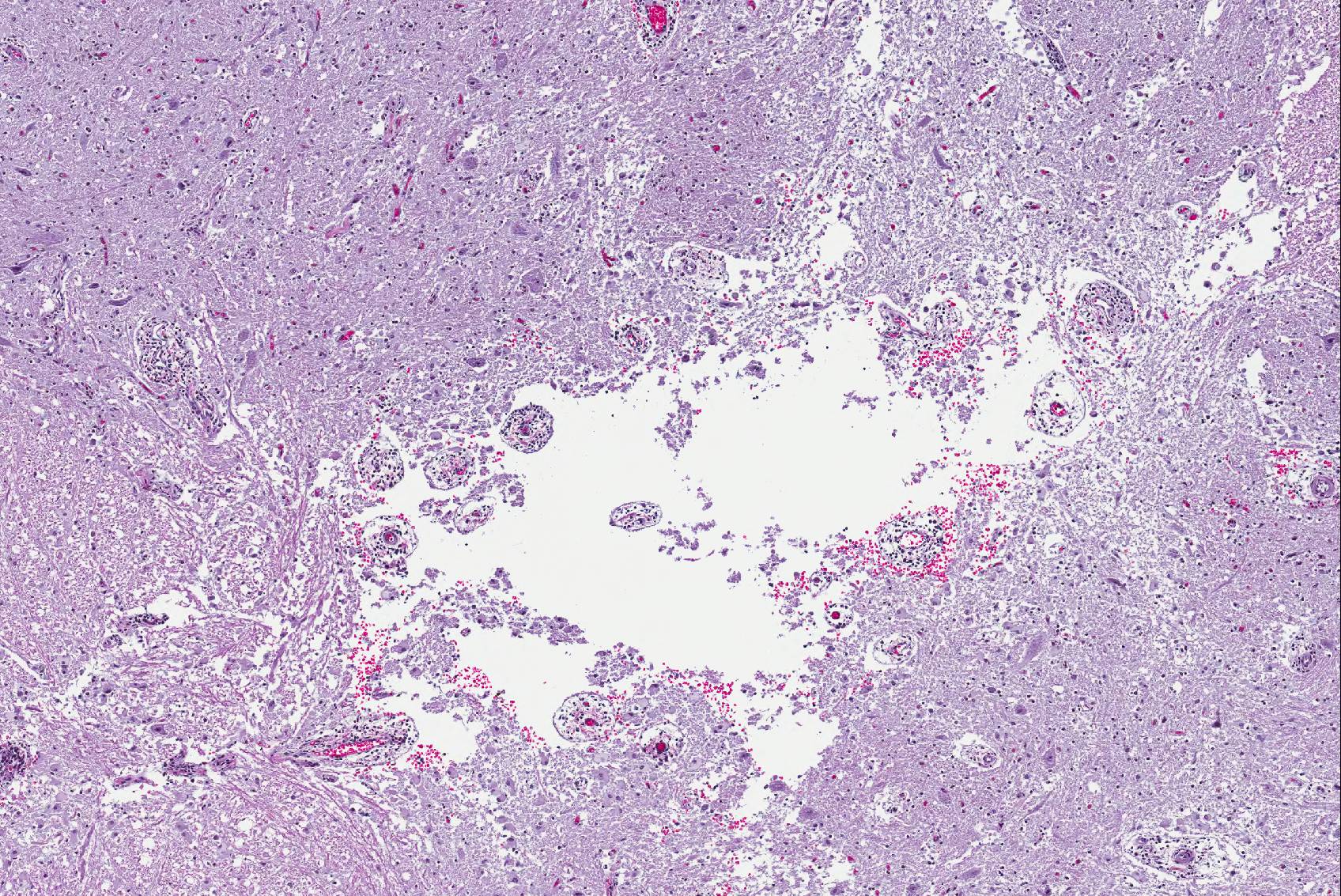
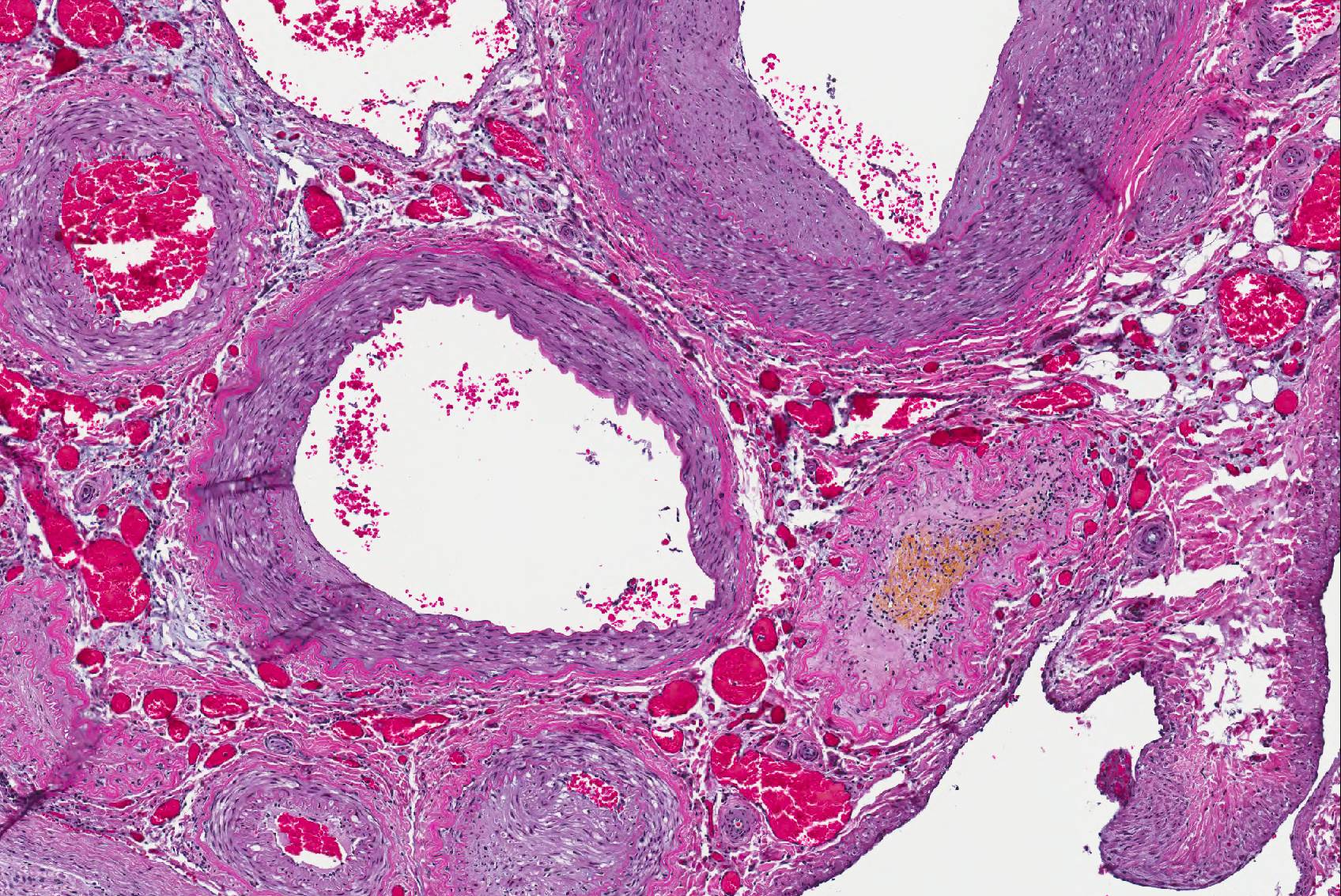
Microscopic Description: Spinal cord: All tissues represented by the slide are affected by variable degrees of inflammation, necrosis and degenerative changes. Diffusely, submeningeal spaces are markedly expanded and disrupted by dense infiltrates composed of copious amounts of lymphocytes admixed with fewer macrophages and plasma cells admixed with fibrin. Mononuclear infiltrates extend into the dura and surround nerve roots and blood vessels (perivascular cuffs). In the spinal cord, most blood vessels throughout the white and gray matter are surrounded by inflammatory cells. In many slides, the central region of gray matter is rarefied and, in severe cases, undergoes liquefactive necrosis. The dorsal root ganglion (displayed on some slides) has multifocal areas of satellitosis associated with chromatolysis and neuronophagia. Multifocally, blood vessels within the vascular plexus are completely occluded by subintimal proliferations of spindle cells, hematoidin deposits or fibrinous thrombi. Throughout the section, numerous blood vessels undergo fibrinoid necrosis.
Contributor’s Morphologic Diagnosis:
Spinal cord: Severe, diffuse, chronic-active lymphocytic meningomyelitis with polyradiculoneuritis, pachymeningitis, vasculitis, and myelomalacia.
Contributor’s Comment: Brucellosis is a zoonotic and endemic disease that affects a diverse array of land and aquatic mammals in many world regions including the Middle East, Asia, Africa, and North and South America. Domestic and wildlife species can become chronically infected, thus playing an important role disseminating the disease and acting as reservoirs4,12.
Brucella are nonmotile, unencapsulated, facultative intracellular, gram-negative coccobacilli. Taxonomically, the Brucella genus is divided into ten species according to their host specificity. Four out the ten, Brucella abortus, B. melitensis, B. suis, and B. canis are pathogenic to humans4.
Their lifecycle contains three phases: incubation, acute and chronic infection. The bacterium enters the host via contact with mucosal surfaces and is phagocytized by macrophages and dendritic cells that reach the lymphatic system. Systemic infection follows replication in peripheral and visceral lymph nodes6. Once in the host phagocytic cell, the bacterium forms the Brucella-containing vacuole (BCV). Derived from the endoplasmic reticulum; the BCV permits the bacteria to evade the immune response, allowing it to survive and replicate with consequent progression to the acute phase where the bacterium infects non-phagocytic cells13.
Brucella have a tropism for the reticuloendothelial system, bone marrow, reproductive organs, and mammary glands, but the central and peripherial nervous system can also be infected6. The pathophysiology behind the initial neurological infection is not completely elucidated. It is known that the inflammation associated with the initial infection is the key contributor to the lesions associated with neurobrucellosis. In the literature, a robust body of evidence shows that, in vivo and in-vitro, Brucella’s lipoproteins infect endothelial and glial cells activating the CNS innate immunity leading to secretion of matrix metalloproteases, nitric oxide, cytokines, and upregulation of Toll-like receptors exacerbating and promoting a severe inflammatory response15.
In marine mammals, Brucella sp. infections were initially reported in 1994, and since then, numerous reports associating cetaceans’ neurological lesions with B. ceti and B. pinnipedialis infection were widely documented14.
In cetaceans, Brucella associated neurological lesions include meningoencephalitis, meningitis, choroiditis, spinal discospondylitis, altered cerebrospinal fluid and remodeling of the occipital condyles2,7,8,9,11.
The incidence of neurological involvement in cetaceans with brucellosis is not known, we speculate that the large vascular plexus inside the cranial vault with anastomosing arteries and veins along the vertebrae and base of the skull, predispose these animals to develop neurobrucellosis. Worthy of note in this case particularly is the pattern of malacia with minimal inflammation in the gray matter. This lesion is broadly characteristic of vascular compromise and ischemia due to the exquisite sensitivity of the gray matter to hypoxia. The extensive vascular involvement observed in this lesion is highly consistent with the gray matter lesion.
The zoonotic potential of marine mammal Brucella sp. is still not clear. There are few reports which describe the isolation of marine mammal Brucella sp from humans exposed to the pathogen that further developed granulomatous lesions. However, characterization and comparison of Brucella sp. Strains from naturally infected marine mammals and isolates from exposed humans differed10,16,18. Therefore, more research is needed to characterize their true zoonotic potential.
Although Brucella sp. is now a well-recognized etiological agent for cetacean’s nonsuppurative neurological disease, other infectious agents are can also develop similar lesions such as Herpesviruses, Toxoplasma gondii, West Nile virus, and Morbillivirus.
JPC Diagnosis:
- Spinal cord: Meningomyelitis, lymphohistiocytic, diffuse, severe with focal grey matter necrosis, lymphohistiocytic radiculoneuritis and fibrinoid vasculitis, Stenella coeruleoalba, dolphin.
- Spinal canal: Arteriosclerosis, proliferative, multifocal, moderate to severe.
Conference Comment: A wide range of marine mammals are exposed to or infected with Brucella sp. The biovars of Brucella sp. that infect marine mammals are genetically distinct from those affecting terrestrial species; however, there have been reported cases of human brucellosis caused by marine mammal serovars3. Clinical disease in cetaceans is most common among marine mammals with pinnepeds being the most sensitive. In cetaceans, Brucella ceti, is the most common infectious culprit resulting in vertebral osteomyelitis and abortions particularly in bottlenose dolphins2. In these cases, atlanto-occipital joints were filled with inspissated, caseous material and there was a chronic, nonsuppurative meningoencephalitis characterized by patchy congestion of meningeal blood vessels and mononuclear cell cuffing of blood vessels in the brain and meninges. A recent article described Brucella spp. Infections in endangered Hector’s dolphins that are currently declining in population1. A total of 27 dolphins found dead on the New Zealand coastline were evaluation for lesions associated with brucellosis. Of note, Brucella pinnipedialis was the most common isolate and resulted in reproductive disease in affected animals which may be a contributing factor to the dwindling numbers in this species.
Conference participants had an energetic dialogue regarding the association of the grey matter necrosis, inflammatory meningoencephalitis, and the lesions in the adjacent arteries; even a temporal thread does not connect these acute, subacute, and chronic lesions, respectively. Atherosclerosis has been reported in marine mammals, specifically aged dolphins, and is most likely an incidental finding in this case17.
Contributing Institution:
Oregon Veterinary Diagnostic Laboratory- College of Veterinary Medicine
Oregon State University, Corvallis, Oregon
http://vetmed.oregonstate.edu/diagnostic
References:
- Buckle K, Roe WD, Howe L, Michael S, et al. Brucellosis in endangered Hector’s dolphins (Cephalorhynchus hectori). Vet Pathol. 2017;54(5):838-845.
- Dagleish M, et al. Isolation of Brucella species from a diseased atlanto-occipital joint of an Atlantic white-sided dolphin (Lagenorhynchus acutus). Veterinary Record. 2007;160(25):876-877.
- Davison NJ, Barnett JEF, Perrett LL, et al. Meningoencephalitis and arthritis association with Brucella ceti in a short-beaked common dolphin. J Wildl Dis. 2013 49(3): 632-636.
- de Figueiredo P, et al. Pathogenesis and immunobiology of Brucellosis: review of Brucella–host interactions. The American Journal of Pathology. 2015;185(6):1505-1517.
- Dold C. Cetacea (whales, dolphins, porpoises). In: Miller RE, Fowler ME, eds. Fowler’s Zoo and Wildlife Medicine. 8. St. Louis, MO: Elsevier; 2015:431, 446-447.
- Franco, M.P., et al. Human brucellosis. The Lancet infectious diseases. 2007;7(12):775-786.
- Godfroid J, et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Veterinary Research. 2005;36(3):313-326.
- González L, et al. Chronic meningoencephalitis associated with Brucella infection in live-stranded striped dolphins (Stenella coeruleoalba). Journal of Comparative Pathology. 2002;126(2-3):147-152.
- Hernández-Mora G, et al. Neurobrucellosis in stranded dolphins, Costa Rica. Emerging infectious diseases. 2008;14(9):1430.
- McDonald W, et al. Characterization of a Brucella strain as a marine-mammal type despite isolation from a patient with spinal osteomyelitis in New Zealand. Journal of Clinical Microbiology. 2006;44(12):4363-4370.
- Nymo IH, Tryland M, Godfroid J. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Veterinary Research. 2011;42(1):93.
- Pappas G, et al. The new global map of human brucellosis. The Lancet infectious diseases. 2006;6(2):91-99.
- Pizarro-Cerdá J, et al. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infection and immunity. 1998;66(5):2387-2392.
- Ross H, et al. Brucella species infection in sea-mammals. Veterinary Record. 1994;134(14):359-359.
- Samartino CG, et al., Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. The American Journal of Pathology. 2010; 76(3):1323-1338.
- Sohn A, et al. Human Neurobrucellosis with intracerebral granuloma caused by a marine mammal. Vet. Rec. 2003;13:1342-1353.
- Sotnikov L. Comparative characteristics of the pathological anatomy of spontaneous atherosclerosis in representative of various classes of animals. Arkh Patol. 1967;29(9):61-67.
- Whatmore AM, et al. Marine mammal Brucella genotype associated with zoonotic infection. Emerging Infectious Diseases. 2008;14(3):517.