WSC Conference 10, Case 2:
Signalment:
1-year-old, female red abalone (Haliotis rufescens)
History:
Four red abalone were acquired from California for placement in an aquarium. Re-ceding tissues were noted in one of the an-imals. The co-housed animal was apparently healthy.
Laboratory Results:
PCR for Xenohaliotis californiensis was positive.
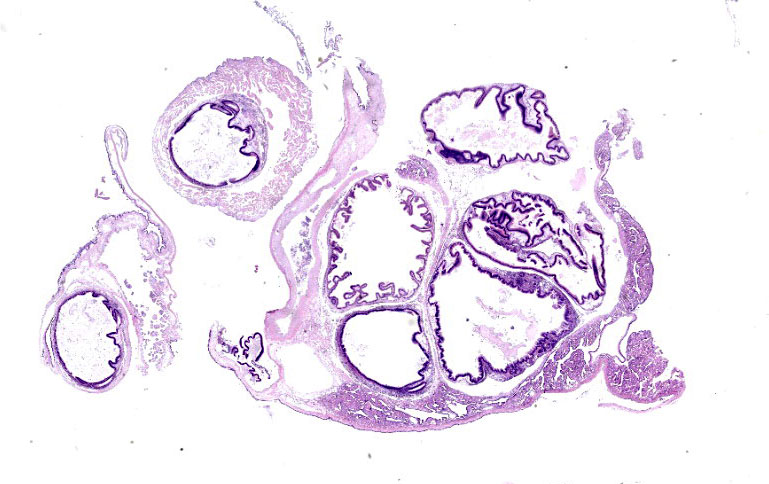
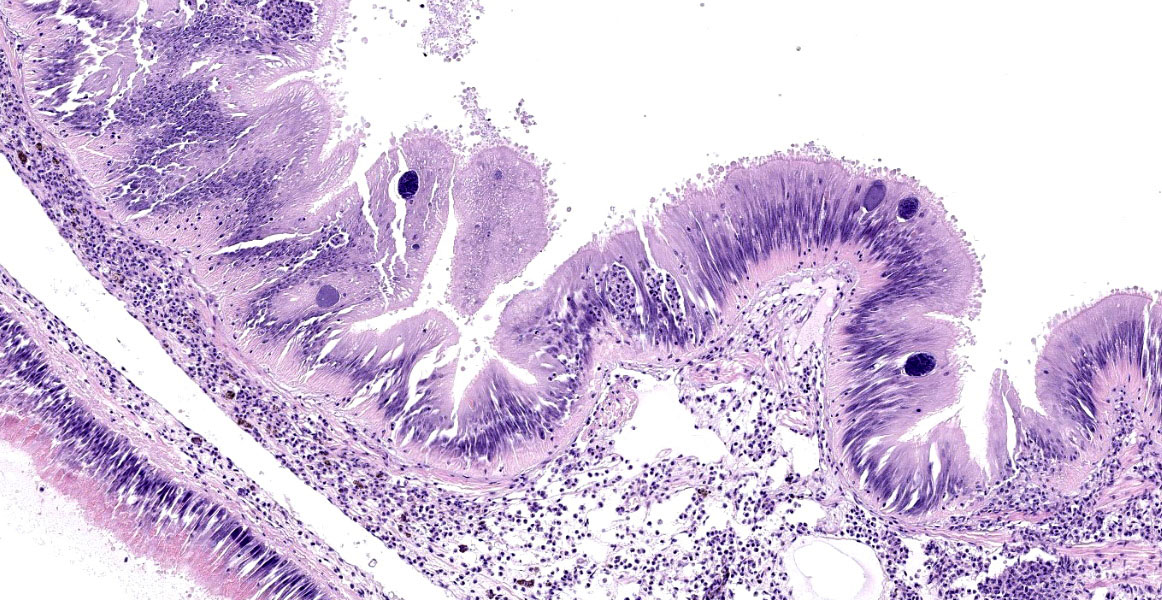
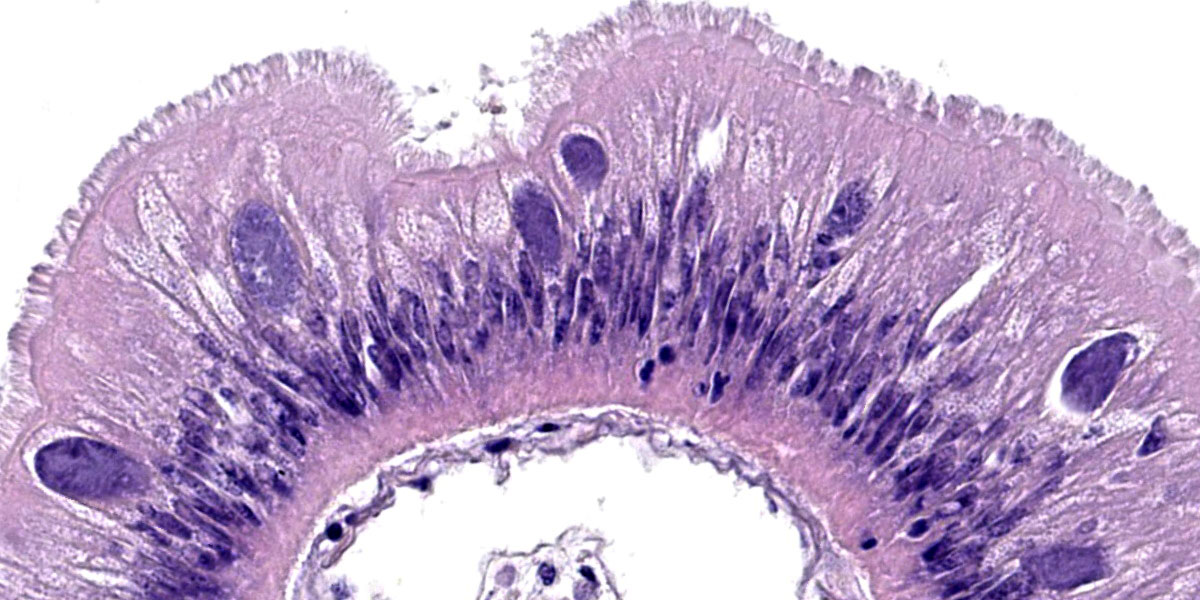
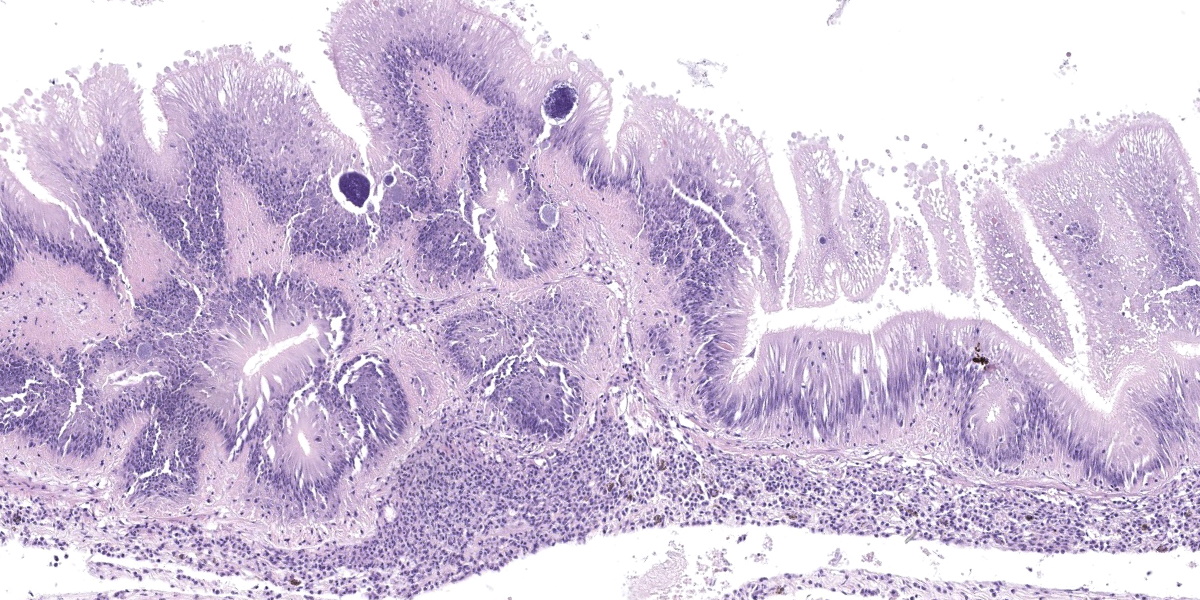
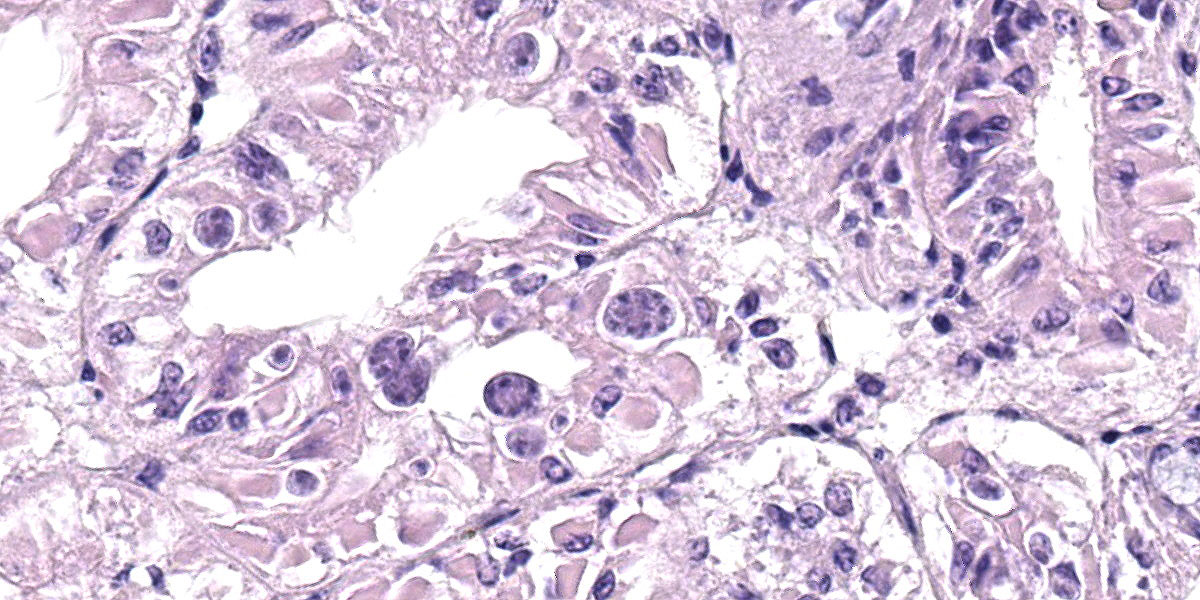
Microscopic Description:
Posterior esophagus: Moderate numbers of epithelial cells lining the posterior esopha-gus contain large (15-20 µm in diameter) basophilic cytoplasmic inclusions. The inclusions range from palely basophilic and homogeneous to moderately basophilic and punctate to deeply basophilic and granular. The majority of inclusions are located in the apical aspect of the epithelial cells. Rarely, there are similar inclusions in the intestinal epithelium. Hucker-Twort gram stain shows the organisms within the inclu-sions to be gram negative.
Contributor’s Morphologic Diagnosis:
Posterior esophagus: Moderate numbers of intracytoplasmic epithelial inclusions con-sistent with rickettsiae (abalone rick-ettsiosis).
Contributor’s Comment:
Inclusions within the digestive tract of this animal are consistent with infection with the rickettsial organism Xenohaliotis cali-forniensis, the agent associated with abalo-ne withering syndrome. As this disease is reportable to the OIE, samples were submit-ted to the NVSL for confirmatory histo-pathology and PCR testing. A PCR product generated with PCR primers proscribed by the OIE was sequenced and showed 99.37% identity with X. californiensis sequences in Gen Bank.4
Abalone withering syndrome affects multiple species within the Haliotis genus in both wild and farmed animals. The causative agent, Xenohaliotis californiensis, pri-marily infects the posterior esophagus and the digestive
gland, but can also be seen to a lesser extent in the intestine.5 Some of the slides submit-ted
here have rare cytoplasmic inclusions pre-sent in intestinal epithelial cells. Sections of the digestive gland from this animal are not present on the submitted slides; addi-tional sections containing the digestive gland examined by the submitter did not exhibit inclusions within digestive gland cells. Infection of the digestive gland leads to degeneration/metaplasia of the digestive tubules which leads to anorexia, depletion of glycogen reserves, and subsequent use of the foot muscle as an energy source.5 Atro-phy of the foot muscle is the characteristic gross change that gives the syndrome its name.
Contributing Institution:
USDA/APHIS
NVSL Pathology Laboratory https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/lab-info-services/sa_about_ nvsl/ct_about_nvsl
JPC Diagnosis:
1. Gut: Epithelial necrosis, single cell, multifocal, moderate, with hemocytic inflammation and intracytoplasmic bac-terial inclusions.
2. Kidney: Coccidiosis, intraepithelial, multifocal, moderate with mild epithe-lial degeneration.
JPC Comment:
Withering syndrome (WS) is a bacterial disease characterized by a severely shrunken body and infection by the organism Xenohaliotis californiensis. The organism is an obligate intracellular bacterium that infects the abalone digestive epithelia, causing the morphologic abnormalities detailed by the contributor and resulting in physiologic starvation.1 The organism is likely spread via direct fecal-oral transmis-sion, with initial infection in the post-esophagus, followed by the intestine, and finally and most devastatingly, the digestive gland.1
WS affects all members of the Haliotis ge-nus with varying degrees of severity, depending on species and environmental conditions. The disease was first observed in black aba-lone populations on the California coast in 1985 after a particularly strong El Niño event caused sustained, increased water temperatures off the western US.1 This pat-tern has held in subsequent years, with the geographic range of clinical WS moving steadily ever northward with increasing coastal water temperatures and episodic high-mortality events associated with El Niño years.
Temperature is the central factor in the ecology of WS with transmission and dis-ease nearly eradicated at water tempera-tures of 12.3oC, but high transmission and extreme clinical signs evident at 18.7oC.1 The susceptibility of Haliotis species also varies widely, with green and pink abalone exhibiting no clinical effects of infection and no mortality, red abalone exhibiting moderate mortality, and black abalone ex-hibiting catastrophic mortality rates of up to 99%.1
Diagnosis of WS requires both identification of the pathogen, either by in situ hybridization or by PCR and sequence analy-sis, and observation of the characteristic histologic changes of pedal and digestive gland atrophy and digestive gland metaplasia. PCR may also be used to detect Xenohaliotis californiensis prior to the movement of animals as part of infectious disease prevention protocols.1 Scrupulous husbandry practices, such as reduced stocking densities, maintaining cool water tempera-tures, disinfection of hands and equipment when moving among groups or tanks of animals, and isolation or culling of infected animals are the mainstays of WS prevention in Haliotis species under human care.
Despite the dramatic mass mortality events associated with WS, certain black abalone populations appear to have developed some resistance to the disease, with progeny of mass mortality event survivors experiencing longer survival times upon infection.2 In a second optimistic development, a bacteri-ophage has been identified within Xeno-haliotis californiensis in WS-affected farmed abalone. The presence of this bacte-riophage effectively eliminates the ability of Xenohaliotis californiensis to cause dis-ease in farmed abalone, though the extent to which the phage is present in the wild is currently unknown.2 More research is cur-rently needed to understand the complex interactions of climate change, water tem-peratures, natural selection pressures, and host-agent-parasite relationships and what these interactions portend for the future of Haliotis species.
Conference participants briefly discussed the anatomy and ecology of the red abalone, which, due to their endangered status, can only be harvested via recreational free diving. Participants also discussed the coccidial organisms noted throughout the kidney. These are most consistent with Margolisiel-la haliotis (formerly called Pseudoklossia haliotis; also called, rather pointedly, aba-lone kidney coccidia) which infects multi-ple species of abalone and targets renal epi-thelial cells. Lesions reportedly associated with this infection include renal epithelial cell hypertrophy and inflammation.3
Participants discussed lesion localization within the abalone digestive tract. The moderator felt it unnecessary to parse the different gastrointestinal segments present on the examined slide, particularly since the term “gut” is used extensively in invertebrate pathology to refer to the entire inver-tebrate tubular alimentary tract. Discussion ended with a quick review of rickettsia-like bacterial diseases of veterinary note, in-cluding Epitheliocystis, Rickettsiella scor-pionisepticum, Neorickettsia risticii, and Neorickettsia helmintheca.
References:
1. Crosson LM, Wight N, VanBlaricom GR, Kiryu I, Moore JD, Friedman CS. Abalone withering syndrome: distribu-tion, impacts, current diagnostic meth-ods and new findings. Dis Aquat Org. 2014;108:261-270.
2. Friedman CS, Wight N, Crosson LM, VanBlaricom GR, Lafferty KD. Re-duced disease in black abalone follow-ing mass mortality: phage therapy and natural selection. Front Microbiol. 2014;5:1-10.
3. Friedmann CS, Gardner GR, Hedrick RP, Stephenson MD, Cawthorn RJ, Up-ton SJ. Pseudoklossia haliotis sp. n. (Apicomplexa) from the kidney of Cali-fornia abalone, Haliotisspp. (Mollusca). J. Invertebr. Pathol. 1995;66:33-38.
4. World Health Organization. Chapter 2.4.8 Infection with Xenohaliotis cali-forniensis In: Aquatic Animal Health Code. 2019:8-9.
5. World Health Organization. Chapter 2.4.8 Infection with Xenohaliotis cali-forniensis In: Aquatic Animal Health Code. 2019:2-3.