Signalment:
13-month-old Hampshire-cross castrated ram (
Ovis aries).The animal had a 5-7 day history of progressive ill thrift, and was subsequently euthanized due to poor quality of life.
Gross Description:
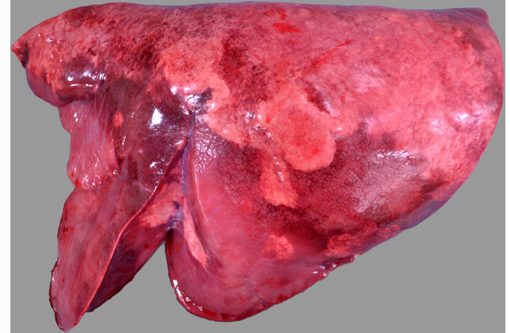
Bilaterally, the cranioventral lung lobes were markedly firm on palpation. Multifocally visible in the immediate subpleural parenchyma of all lung lobes were numerous, coalescing, white, non-raised, nodular foci. On cut section the parenchyma was consolidated, mottled pale tan to dark red to brown, and displayed an increased prominence of cross-sectional profiles of small to medium caliber airways due to circumscription by dense cuffs of moderately firm white tissue.
Histopathologic Description:
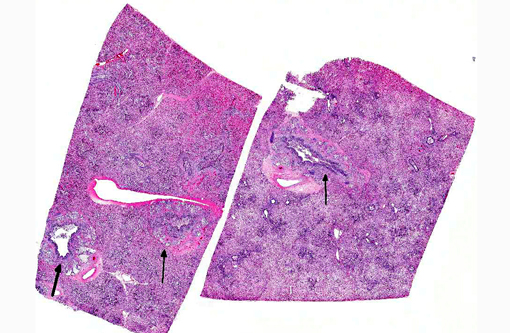
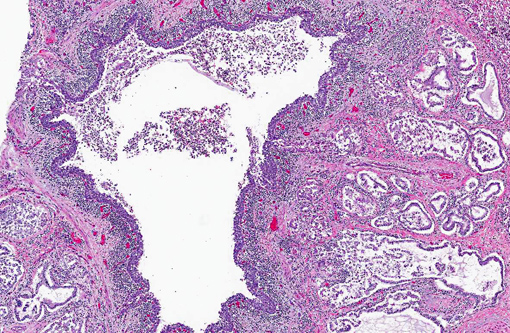
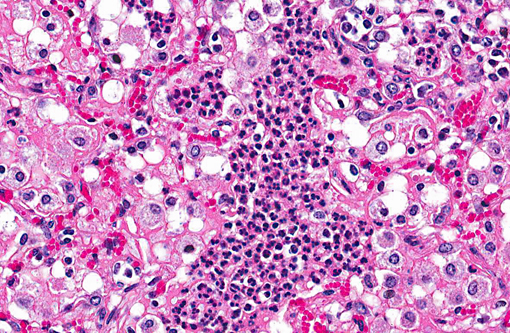
Lung: Affecting 60-100% of the parenchyma within submitted sections, alveolar lumina are variably filled by large numbers of neutrophils and macrophages admixed with marked amounts of edema, and lesser amounts of fibrin. Frequently, macrophages contain abundant foamy cytoplasm, with rare multi-nucleate giant cells present. Multifocally, moderate numbers of affected alveoli are lined by plump, cuboidal epithelial cells (type II pneumocyte hyperplasia). Diffusely, the lumens of bronchi and bronchioles are also filled with variable numbers of neutrophils admixed with fewer macrophages, sloughed epithelial cells, moderate amounts of fibrin, and rare clusters of coccobacilli. Frequently, the submucosa of bronchi and bronchioles, as well as the adventitia of blood vessels, are expanded by cuffs of lymphocytes and plasma cells up to 10 cell layers thick, occasionally which are arranged in more follicular aggregates. Segmentally, there is hyperplasia of the respiratory epithelium of affected bronchi and bronchioles characterized by crowding and piling up of epithelial cells.Â
Multifocally, interlobular septa are also expanded by ectatic lymphatics, edema, and lesser amounts of fibrin. Within some sections, rare bronchiolar lumens are partially occluded by nodular protrusions of fibrous connective tissue which are lined by hypertrophied epithelial cells (nodular hyaline casts).Â
Morphologic Diagnosis:
Lung; bronchopneumonia, suppurative and histiocytic, chronic-active, diffuse, severe, with bronchial epithelial and type II pneumocyte hyperplasia, and perivascular and peribronchiolar lymphofollicular proliferation.
Lab Results:
PCR of lung tissue was positive for
Mycoplasma spp. and was confirmed as
Mycoplasma ovipneumoniae by genetic sequencing. PCR for Maedi-Visna virus (MVV; Ovine Progressive Pneumonia) was negative. Routine aerobic culture from the lung yielded heavy growth of
Mannheimia haemolytica.
Condition:
Mycoplasma ovipneumoniae
Contributor Comment:
Numerous
Mycoplasma spp. have been documented to cause disease in small ruminants including
M. mycoides ssp.Â
Mycoides, large colony type (LC),
M. mycoides ssp.Â
capri, M. capricolum ssp.Â
capricolum, M. putrefaciens, M. agalactiae, M. capricolum ssp.Â
capripneumoniae (the causative agent of contagious caprine pleuropneumonia (CCPP), and
M. ovipneumoniae.(2) Similar to cattle, other domestic animal species, and laboratory rodents, these mycoplasmas cause a wide range of pathologic conditions not only including pneumonia/pleuropneumonia, but also polyarthritis, septicemia/polyserositis, keratitis/conjunctivitis, and mastitis.(2,5,10) Specifically in sheep,
Mycoplasma ovipneumoniae is the primary etiologic agent involved in the condition known as chronic enzootic pneumonia or chronic non-progressive pneumonia, a multi-factorial disease complex which typically affects lambs of 1 year of age or less.(2,5,10,12)
M. ovipneumoniae infection is typically subclinical, frequently only causing a mild, non-fatal pneumonia that results in poor growth rates, until it becomes compounded by additional risk factors such as stress, poor air quality, adverse weather conditions, respiratory syncytial or parainfluenza viral infections, and secondary bacterial infections, which combine to cause overt clinical respiratory disease.(2,5,10,12)
The spectrum of histological lesions present in this animal are a good representation of the pathogenesis of this disease syndrome, as the prominent lymphoid cuffing and bronchial epithelial hyperplasia are consistent with histological findings previously reported for
M. ovipneumoniae, and typify mycoplasmal pneumonias, while the suppurative inflammation is reflective of the secondary
Mannheimia haemolytica infection and not of
M. ovipneumoniae infection.(2,12) Besides
M. haemolytica, other secondary bacterial infections reported with ovine pulmonary mycoplasmosis include
Pasteurella multocida and
Bibersteinia trehalosi (formerly
Pasteurella haemolytica biotype T).(5) In addition to domestic sheep,
M. ovipneumoniae has also been reported to cause similar lesions in Bighorn sheep (
Ovis canadensis canadensis), as well as predispose them to secondary, fatal
M. haemolytica pneumonia.(1,3)
Colonization of the ciliated respiratory epithelium and induction of ciliostasis are shared pathogenic features of
Mycoplasma spp. pneumonia, factors which are thought, at least in part, to prevent clearance of the organism from the respiratory tract by the innate defense system of the mucociliary escalator.(4,7) While unique, these features are also characteristic of
Bordetella bronchiseptica and cilia-associated respiratory bacillus (CAR bacillus), both important bacterial causes of respiratory disease in numerous laboratory and domestic animal species.(10,11) Specifically, investigations into the ability of
M. ovipneumoniae to persist in the respiratory tract of sheep have shown that this persistence may be due to a combination of: 1) an initial aberrant immune response to the organism and 2) delayed generation of a protective systemic humoral immune response.(9) Experimental evidence corroborating this includes the presence of ciliary autoantibodies in acutely infected sheep, and the resolution of late clinical disease following systemic generation of antigen-specific IgG antibodies.(8,9) It is speculated that this delayed development of protective humoral immunity results from a marked variation in antigenicity between organisms, as well as expression of a polysaccharide capsule.(4,9)
JPC Diagnosis:
Lung: Bronchopneumonia, neutrophilic and histiocytic, focally extensive, moderate with peribronchial, peribronchiolar, and perivascular lymphoplasmacytic proliferation.
Conference Comment:
A tissue Gram stain revealed moderate numbers of gram-negative coccobacilli within peribronchial glands, interpreted as secondary bacterial colonization. Although none were isolated, conference participants speculated upon the presence of an initiating viral agent, which may have created ideal physiologic conditions for subsequent bacterial infection. Potential etiologic agents include bovine parainfluenza virus 3, bovine respiratory syncytial virus, Maedi-Visna virus, and (less likely) peste des petits ruminants virus. Both bovine parainfluenza virus 3 and bovine respiratory syncytial virus are members of the family
Paramyxoviridae. Alone, they are unlikely to cause respiratory disease; however, they are commonly associated with both shipping fever and bovine respiratory disease complex, which frequently induce secondary bronchopneumonia due to bacterial agents such as
Mannheimia haemolytica. Both of these syndromes are characterized by respiratory signs such as nasal discharge, tachypnea, anorexia, fever and general malaise, as exhibited in this case.(6) Maedi-Visna virus, the cause of ovine progressive pneumonia, is a lentivirus from the family
Retroviridae, which is closely related to caprine arthritis-encephalitis virus.(6) Discovered in Iceland, this disease was historically distinguished by clinical signs indicating both respiratory and central nervous system lesions (
maedi means dyspnea and
visna means fading away in Icelandic); however, currently, the most common presentation is a slowly progressive pneumonia, which often predisposes secondary bacterial infection.(2) Peste des petits ruminants virus is a morbillivirus which, although not endemic to North America, can also cause respiratory disease in small ruminants.(6)
In this case, PCR analysis implicates
Mycoplasma ovipneumoniae as the inciting agent. Mycoplasmas, the smallest known bacteria, are obligate parasites that lack a cell wall and have a protein- and lipid-rich plasma membrane. They have a relatively small genome and a propensity for genomic rearrangement, leading to frequent variations in cell surface antigens. These characteristics likely result in an innate ability to evade the host immune response. In addition to ciliostasis, other pathogenic mechanisms of mycoplasmas include alteration of prostaglandin synthesis and induction of lymphocyte apoptosis. Additionally, mycoplasmal membranes contain superantigens, which generate a substantial nonantigen-specific immune response; this is the likely cause of the characteristic peribronchiolar lymphoid cuffing often associated with pulmonary mycoplasmosis.(2) Superantingens bind the V
+�-� domain of the T-lymphocyte receptor (TCR) with the α-chain of a class II major histocompatibility complex (MHC). This occurs outside of the normal antigen binding site and results in polyclonal T-lymphocyte activation regardless of antigen specificity, as well as massive cytokine release (
see 2013-14 WSC conference 1, case 3). In lambs, infection with
M. ovipneumoniae and
M. arginini can induce paroxysmal coughing of such severity as to induce rectal prolapse, known as coughing syndrome.(8)
References:
1. Besser TE, Cassirer EF, Potter KA, et al. Association of Mycoplasma ovipneumoniae infection with population-limiting respiratory disease in free-ranging Rocky Mountain bighorn sheep (Ovis canadensis canadensis). J Clin Microbiol. 2007;46:423-430.Â
2. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier; 2007:579-650.
3. Dassanayake RP, Shanthalingam S, Herndon CN, et al. Mycoplasma ovipneumoniae can predispose bighorn sheep to fatal Mannheimia haemolytica pneumonia. Vet Microbiol. 2010;145:354-359.Â
4. Howard CJ, Taylor G. Immune responses to mycoplasma infections of the respiratory tract. Vet Immunol Immunopathol. 1985;10:3-32.
5. Lopez A. Respiratory system, mediastinum, and pleurae. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:458-538.Â
6. MacLachlan NJ, Dubovi EJ, eds. Fenners Veterinary Virology. 4th ed. London, UK: Elsevier; 2011:267-268,308-323.
7. Minion FC. Molecular pathogenesis of mycoplasma animal respiratory pathogens. Front Biosci. 2002;7:1410-1422.
8. Niang M, Rosenbusch RF, Andrews JJ, Lopez-Virella J, Kaeberle ML. Occurrence of autoantibodies to cilia in lambs with a 'coughing syndrome'. Vet Immunol Immunopathol. 1998;64:191-205.
9. Niang M, Rosenbusch RF, Lopez-Virella J, Kaeberle ML. Differential serologic response to Mycoplasma ovipneumoniae and Mycoplasma arginini in lambs affected with chronic respiratory disease. J Vet Diagn Invest. 1999;11:34-40.
10. Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, IA: Blackwell Publishing; 2007:64, 132, 141-143, 211, 226-228, 267-268.
11. Schoeb TR, Davidson MK, Davis JK. Pathogenicity of cilia-associated respiratory (CAR) bacillus isolates for F344, LEW, and SD rats. Vet Pathol. 1997;34:263-270.
12. Sheehan M, Cassidy JP, Brady J, et al. An aetiopathological study of chronic bronchopneumonia in lambs in Ireland. Vet J. 2007;173:630-637.Â