WSC 2022-2023
Conference 18
Case I:
Signalment:
Goitered gazelle (Gazella subguttorosa), adult, gender unknown.
History:
Lung tissue was collected from a carcass found in Darvi soum, Khovd province, Mongolia in January 2017. Formalin-fixed tissue was embedded in paraffin at the State Central Veterinary Laboratory, Mongolia.
Gross Pathology:
The carcass was emaciated.
Laboratory Results:
The lateral flow device penside test for eye swab was positive for Peste-des-petits-ruminants virus antigen.
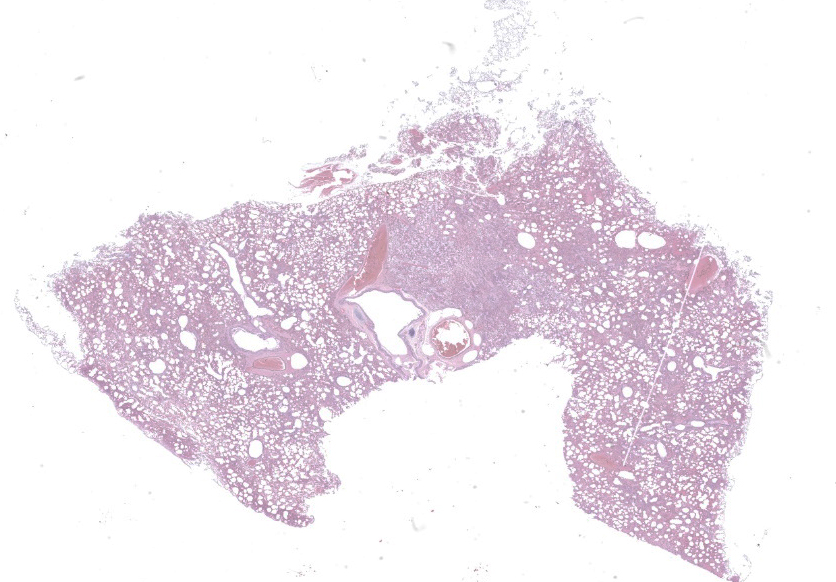
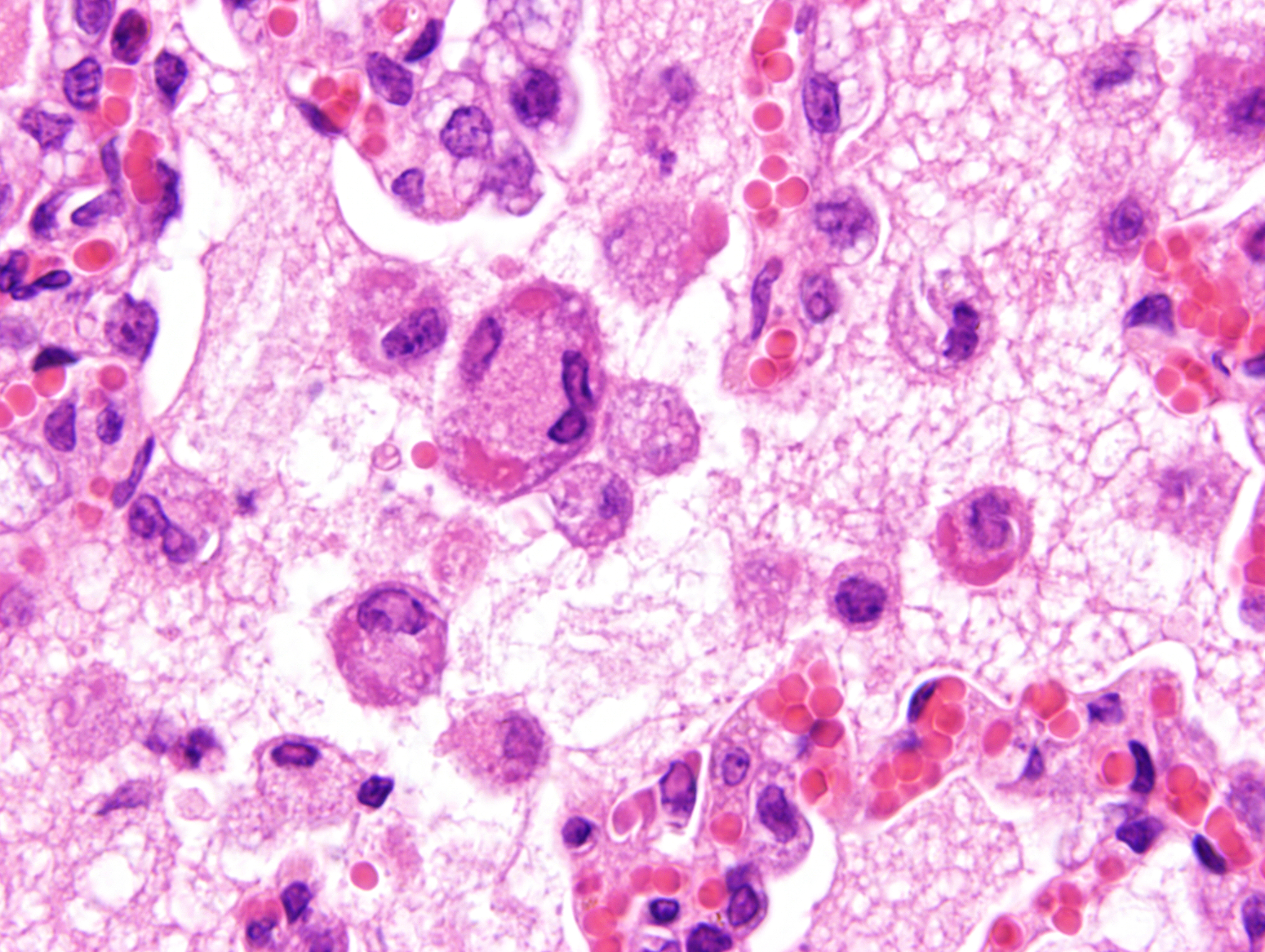
Microscopic Description:
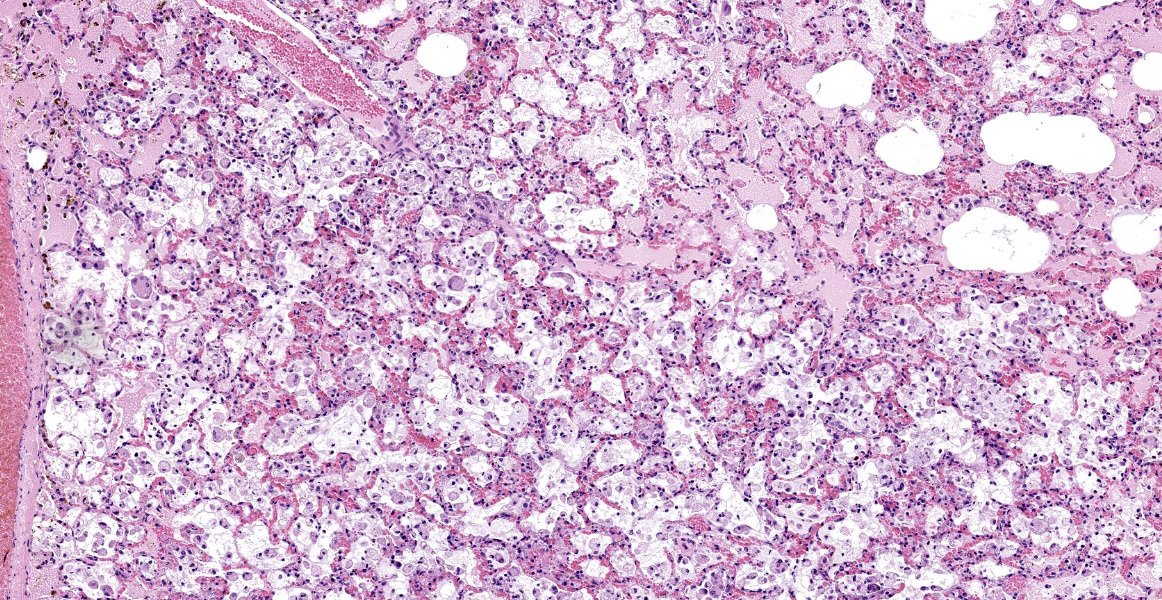
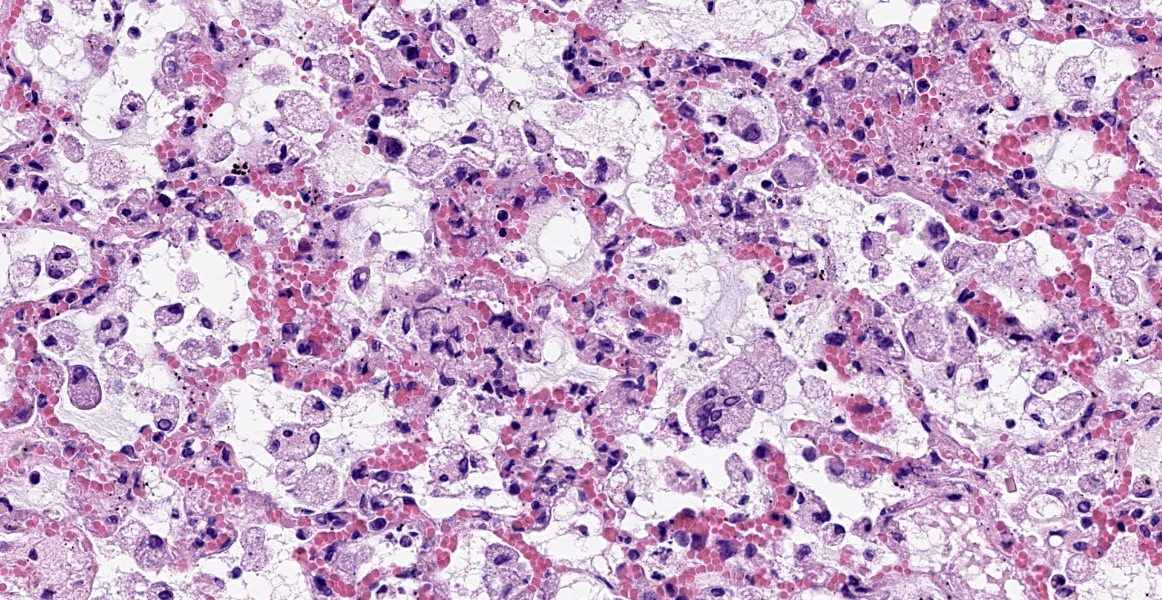
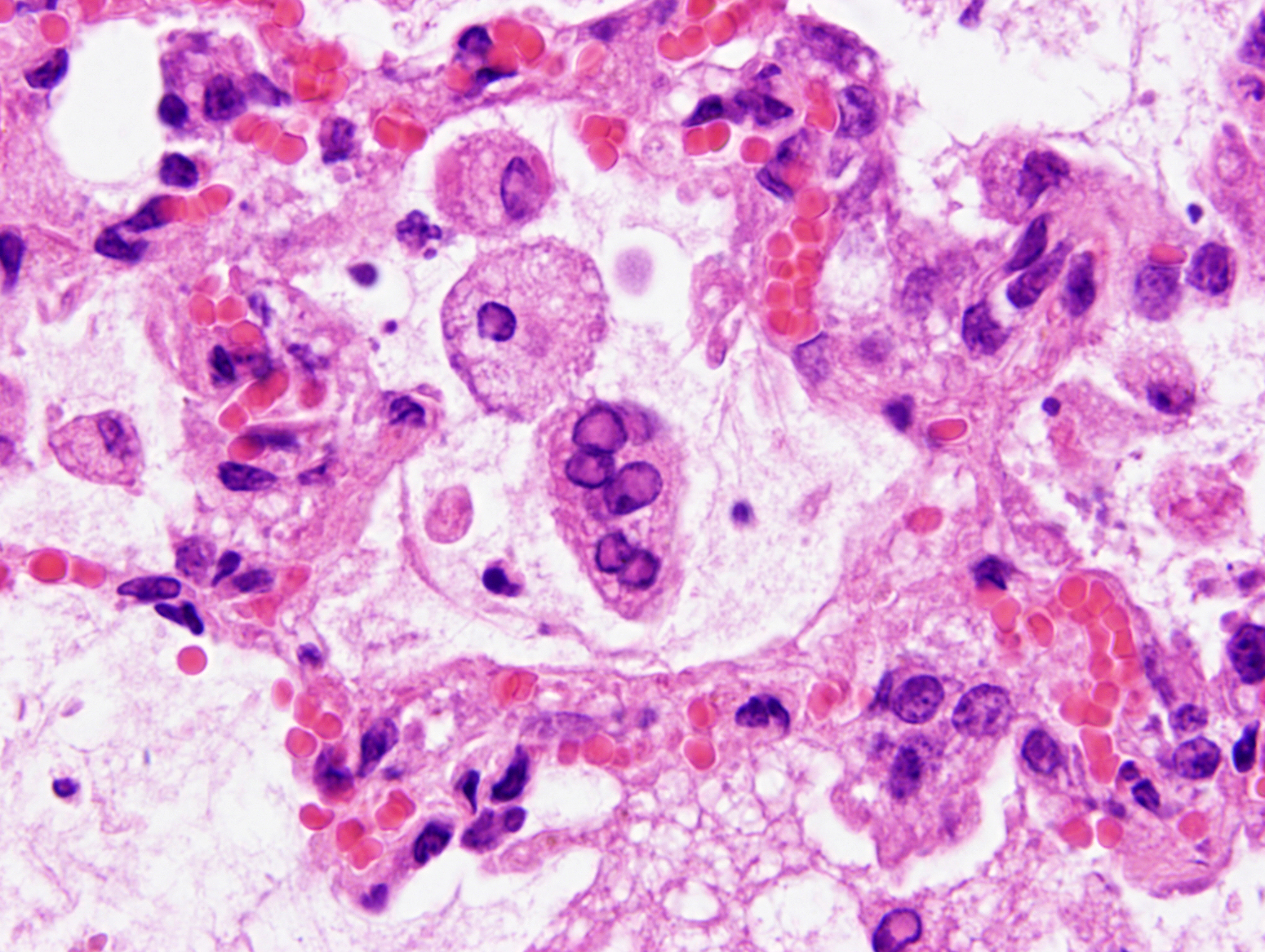
Mild epithelial desquamation and peribronchiial edema are observed in the bronchi. Epithelial necrosis and desquamation are severer in bronchioles. Infiltration of neutrophils, epithelioid macrophages and syncytial cells occurs multifocally in alveolar lumina, which is associated with the proliferation of type II pneumocytes. Bronchial and bronchiolar epithelial cells sometimes contain intracytoplasmic and intranuclear inclusion bodies. Intracytoplasmic and intranuclear inclusion bodies are also frequently observed in epithelioid macrophages and syncytial cells.
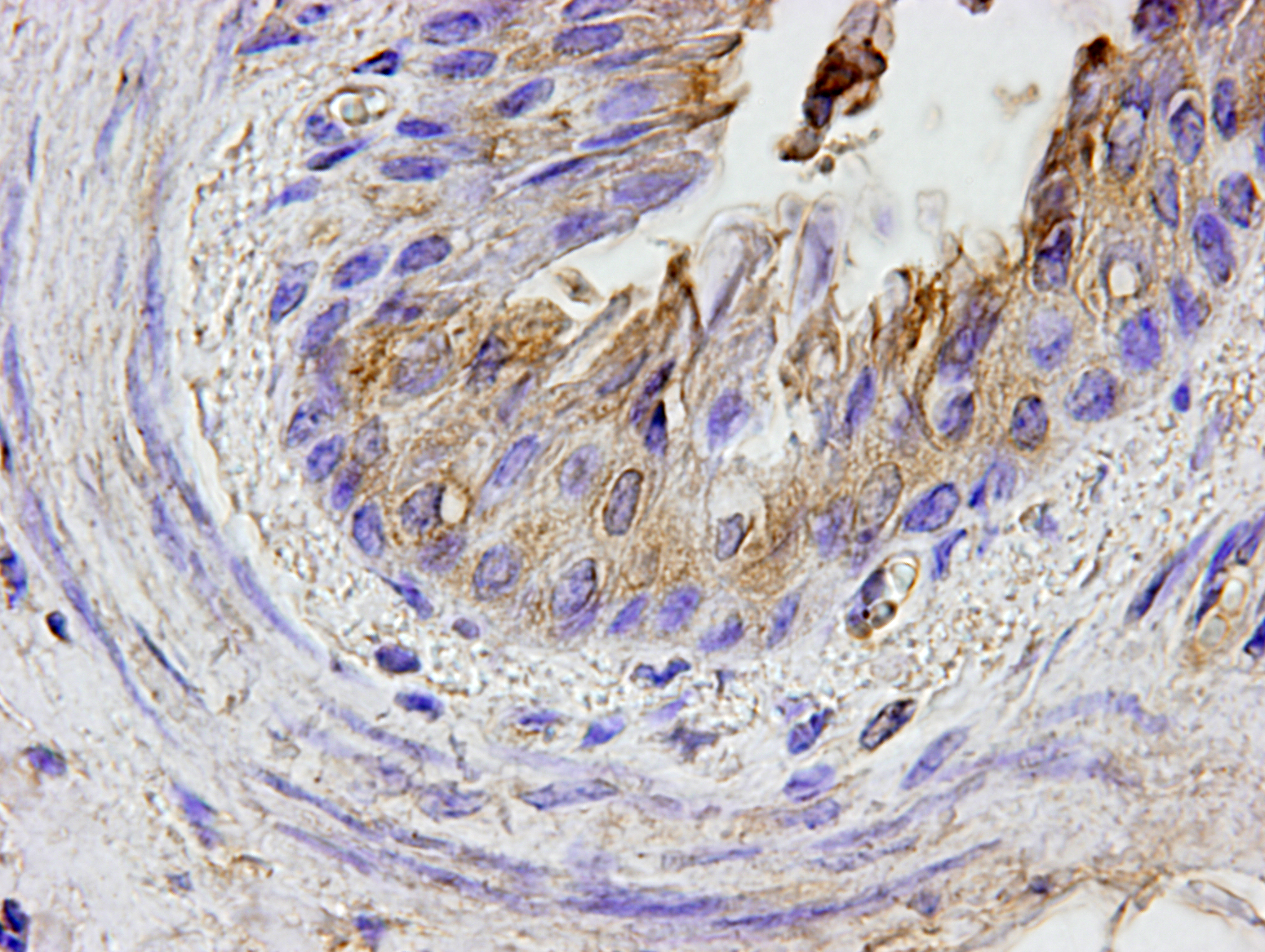
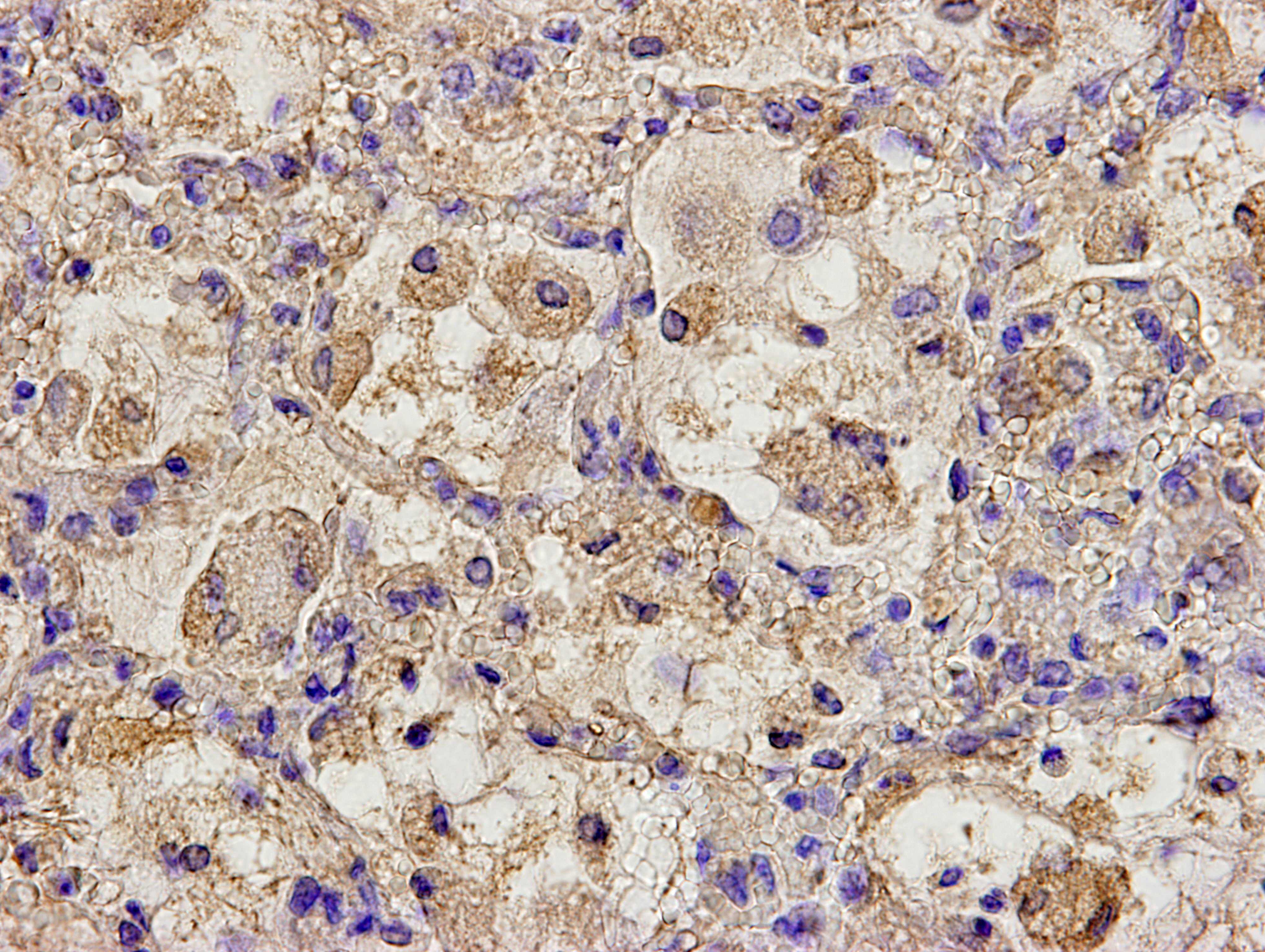
By immunohistochemistry, bronchial and bronchiolar epithelial cells, epithelioid macrophages, syncytial cells, and type II pneumocytes reacted weakly with anti-canine distemper virus monoclonal antibody (clone DV2-12).
Contributor’s Morphologic Diagnoses:
Lung: Acute necrotizing bronchopneumonia with intracytoplasmic and intranuclear inclusion bodies and syncytial cell formation.
Contributor’s Comment:
Peste des petis ruminants (PPR) is a viral disease of goats and sheep characterized principally by stomatitis, diarrhea, oculonasal discharge, and pneumonia. The causative agent is closely related to rinderpest virus and these two are classified along with the measles virus and CDV in the genus Morbillivirus in the Family Paramyxoviridae. PPR was reported to the OIE, for the first time in Mongolia, in 2016 and was confirmed to affect sheep, goat and yak in the western part of Mongolia.2 Death in these domestic animals declined rapidly after extensive vaccination; however, the virus continued to spread to wild animals such as saiga, Siberian ibex and goitered gazelle.2
Histopathological findings observed in this case are similar to those in goats and sheep experimentally infected with PPR virus.1,3 Syncytial cells observed within alveolar spaces were reported to be macrophage origin.3
Contributing Institution:
Laboratory of Comparative Pathology
Faculty of Veterinary Medicine, Hokkaido University
Kita 18, Nishi 9, Kita-ku
Sapporo 060-0818, Japan
https://www.vetmed.hokudai.ac.jp/en/
JPC Diagnosis:
Lung: Pneumonia, bronchointerstitial, necrotizing, diffuse, marked, with edema, numerous viral syncytia, and intranuclear and intracytoplasmic eosinophilic viral inclusions.
JPC Comment:
This case of peste des petits ruminants virus infection in a goitered gazelle bears striking similarities to the case of canine distemper virus infection and pneumonia in Conference 17, Case 2, of this year, as they are both morbilliviruses and produce similar diseases. Peste des petits ruminants (PPR), which translates to mean plague of small ruminants, is known by several other names, including kata, stomatitis-pleuropneumonia complex, goat plague, ovine rinderpest.4,8 Goats and sheep are the primary hosts, with goats typically experiencing more severe disease, and while other animals such as cattle, buffalo, and pigs can be infected, they are considered dead end hosts that do not shed the virus and generally do not develop clinical signs.5
PPR was first described in 1942 by Gargadennec and Lalanne in the Ivory Coast, West Africa, though the disease may have been present beforehand and misidentified as rinderpest virus.5 It is now endemic in Africa, the Middle East, China, India, and south Asia. There are four lineages of the virus, and while historically lineages I, II, and III have been present in Africa and IV present in Asia, lineage IV has now become the dominant strain in Africa.5 Increased incidence has been associated with the spread of lineage IV, possibly indicating that is more virulent.5
PPRV is a nonsegmented, single-stranded, negative-sense morbillivirus, and like other morbilliviruses, is highly lymphotropic and epithliotropic.4,5 The virus uses the hemagglutinin protein H to bind to the typical morbillivirus receptors: signaling lymphocyte activation molecule (SLAM)/CD150 on lymphocytes, macrophages, and dendritic cells; and Nectin-4 on epithelial cells.4,5 The F protein facilitates fusion of the viral envelop and host cell membrane, and the virus subsequently replicates in the cytoplasm.5 The M protein facilitates viral egress from host cells via budding.5
Infection occurs through direct contact with aerosolized virus or infected bodily secretions. It is presumed that the virus first infects leukocytes in the respiratory mucosa and then spread to local lymphoid tissue where initial replication occurs. After an initial immune cell proliferation, viremia results in systemic spread of the virus, and subsequent leukopenia with reduction of CD4+ T cells occurs approximately 4 days post infection. PPRV causes clinically significant immunosuppression by inducing leukopenia and inhibition of type I and II interferon activity due to viral nonstructural proteins.5
PPRV has high morbidity and mortality rates, though certain breeds are more resistant and virulence varies by viral strain.5 The disease has a more rapid onset and clinical progression than rinderpest.8 Clinical signs begin around 3 days post-infection and initially consist of pyrexia, malaise, and serous to mucopurulent oculonasal discharge, and pseudomembranous ulcerations in the mouth and nose, followed by acute severe gastroenteritis and hemorrhagic colitis.5,8 Pneumonia occurs late in the disease process and results in dyspnea.4 In animals which recover, clinical signs resolve by 14 days post-infection, and these animals generally have life-long immunity.4
Profound immunosuppression can lead to secondary bacterial infection by microbes such as Mycoplasma or Pasteurella.4,8 This week’s moderator, Dr. Kali Holder from the Smithsonian’s National Zoo, described how morbilliviruses cause immunosuppression by causing leukopenia, by targeting mechanisms of innate immunity, and by inducing immune amnesia by specifically targeting T and B memory cells and dendritic cells.
Histologic features of PPRV infection are typical for morbilliviruses. Syncytia form in leukocytes of the lymph nodes, white pulp, GALT, oral mucosa, pulmonary alveoli, and liver.4,8 Eosinophilic cytoplasmic and nuclear inclusions occur in the renal pelvis, abomasum, respiratory epithelium, and type II pneumocytes.8 Histologic lesions in the lung include inflammation and necrosis of airway epithelium, bronchointerstitial pneumonia, and type II pneumocyte hyperplasia, as seen in this case.1,8
This week’s moderator also described how PPRV has caused massive mortality events in hoofstock in Mongolia, including the Siberian ibex, the goitered gazelle, and in the critically endangered saiga antelope. Researchers using distance sampling techniques estimate that PPRV outbreak caused a population decline of up 80% in the Saiga antelope.6
References:
- Brown CC, Mariner JC and Olander HJ. An immunohistochemical study of the pneumonia caused by peste des petits ruminants virus. Vet Pathol. 1991;28(2):166-70.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th St. Louis, MO: Elsevier Ltd; 2016:557.
- Kock RA. Mongolia Investigation of Peste des Petits Ruminants (PPR) among wild animals and its potential impact on the current PPR situation in livestock. Mission Report 20th January-1st February 2017. Crisis Management Centre-Animal Health (CMC-AH). 30 March 2017.
- Kumar N, Maherchandani S, Kashyap SK, et al. Peste Des Petits Ruminants Virus Infection of Small Ruminants: A Comprehensive Review. Viruses. 2014;6: 2287-2327.
- Parida S, Muniraju M, Mahapatra M, Mutchuchelvan D, Buczkowski H, Banyard AC. Peste des petits ruminants. Vet Microbiol. 2015; 181(1-2):90-106.
- Pruvot M, Fine AE, Hollinger C, et a. Outbreak of Peste des Petits Ruminants among Critically Endangered Mongolian Saiga and Other Wild Ungulates, Mongolia, 2016-2017. Emerg Infect Dis. 2020; 26(1):51-62.
- Truong T, Boshra H, Embury-Hyatt C, Nfon C, Gerdts V, Tikoo S, Babiuk LA, Kara P, Chetty T, Mather A, Wallace DB, Babiuk S. Peste des petits ruminants virus tissue tropism and pathogenesis in sheep and goats following experimental infection. PLoS One. 2014;9(1):e87145.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier Ltd; 2016:115,130-131.