Signalment:
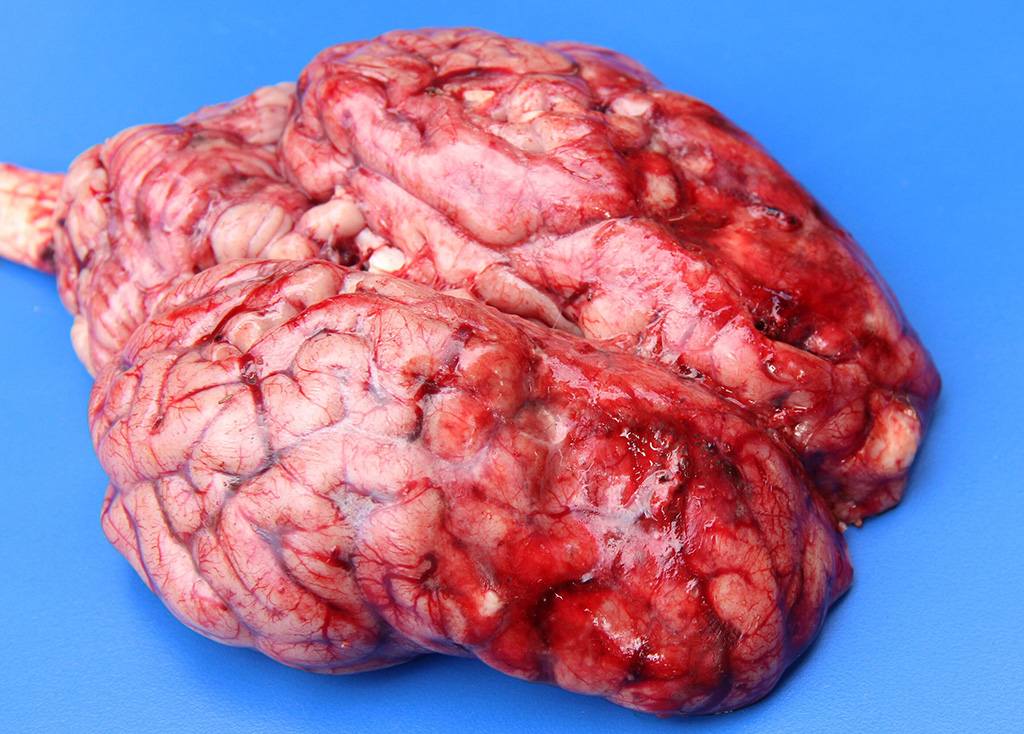
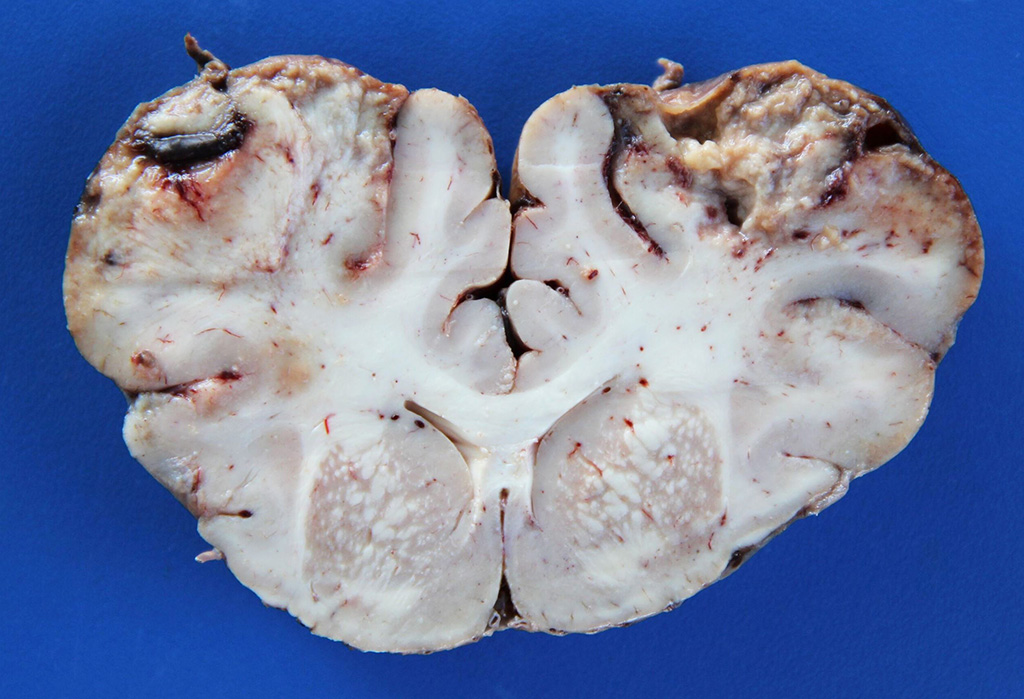
Gross Description:
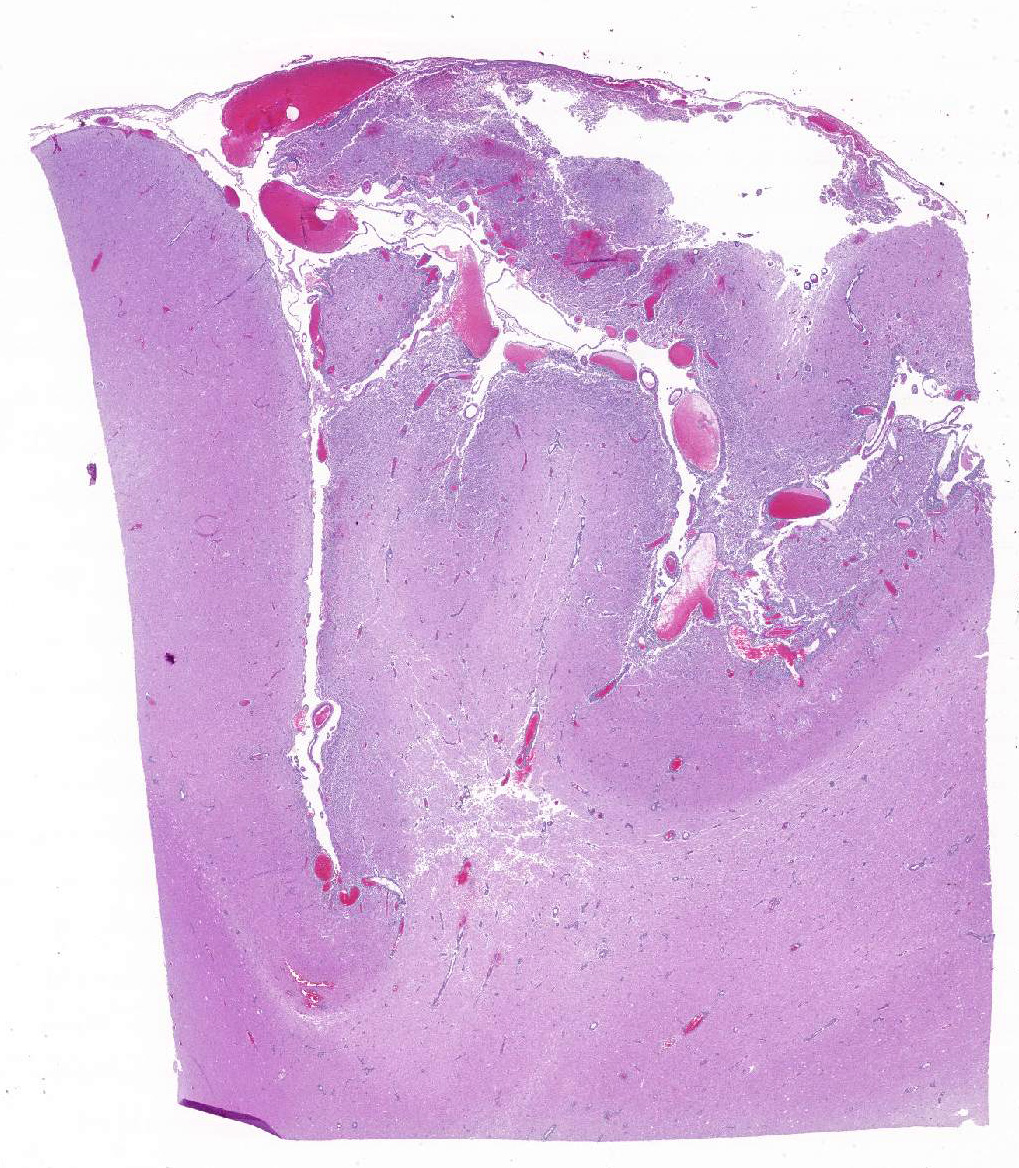
Histopathologic Description:
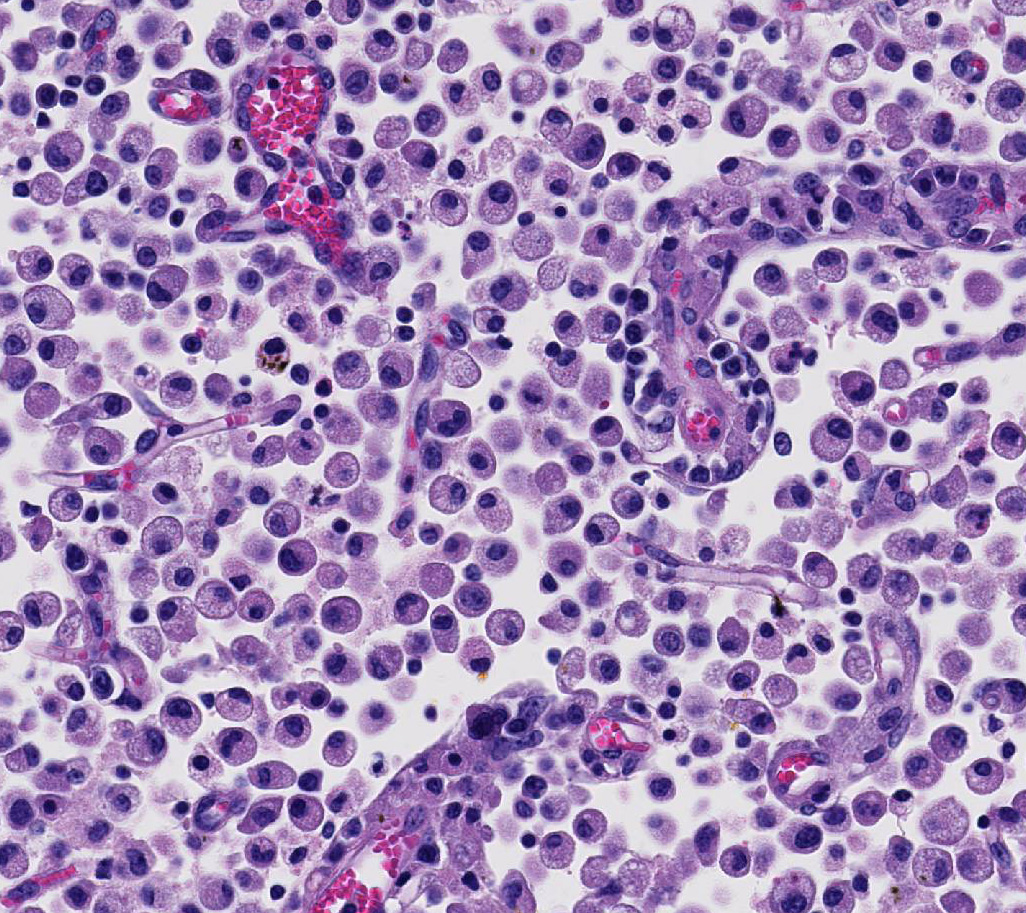
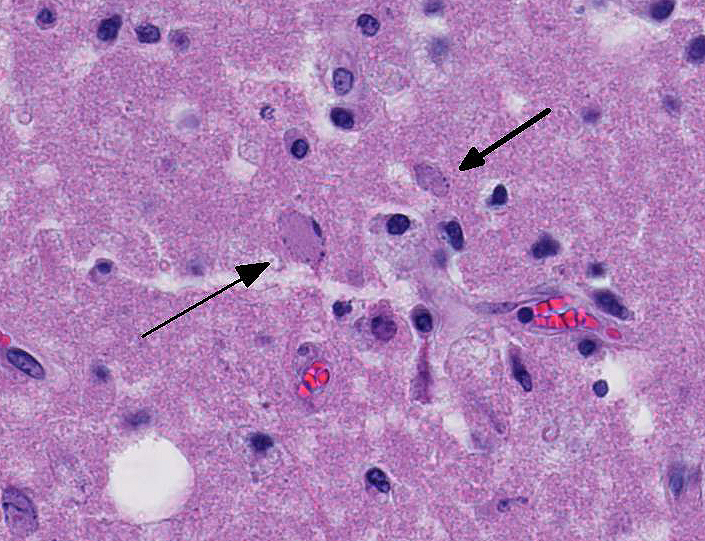
Occasional astrocytes contain large intranuclear amphophilic viral inclusions that pushed the chromatin peripherally. The neuropil within the necrotic areas is sprinkled with basophilic, granular chromatin-like material. Multifocal narrow ring hemorrhages are seen perivascularly and extend into the neuropil. The subcortical white matter is loose due to edema and also diffusely infiltrated by gitter cells. Similar perivascular cuffing is present in both gray and white matter. Perivascular cuffing is also present in the occipital cortex, mesencephalon, pons, medulla and cerebellum.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Meningoencephalitis due to BoHV-1 or BoHV-5 shares many similarities regarding epidemiological and clinicopathological findings, and the diagnostic confirmation should be performed using FAT, viral isolation.8,9 However, the differentiation between neurological infection caused specifically by BoHV-1 or BoHV-5 can only be achieved through the use of molecular ancillary tests such as the glycoprotein Cbased PCR.8,9 Results obtained from studies using this latter method of differentiation in south Brazil showed that BoHV-1 and BoHV-5 may be interchangeably involved in very similar clinicopathological conditions that were previously attributed to a single strain (either BoHV-1 or BoHV-5).8,9 These tests are used only for research purposes in our diagnostic routine, and the con-firmation of bovine herpesviral infection by either FAT or viral isolation is usually sufficient for confirmation of herpesviral infection (but not viral typification) in the diagnostic routine service. In the present case, none of these ancillary tests were available.
The clinical course in cases of meningoencephalitis due to BoHV varies from 1 to 15 days, and affected cattle may develop a wide range of clinical signs such as severe depression, serous ocular and nasal discharge, grinding teeth, circling, blindness, incoordination, head pressing, nystagmus, recumbence, paddling, opistho-tonus, and seizures.5-8 Based on the geographical location and gross findings in this calf, the main differential diagnoses at the time of necropsy included infection by bovine herpesvirus (BoHV-1 or BoHV-5) meningoencephalitis, polioencephalomalacia (PEM), and rabies.7 Severe cases of PEM may rarely present with gross lesions that resemble those described for this calf. Gross findings in cases of bovine rabies are usually absent.5-7 Necrotizing meningoencephalitis due to BoHV affects mainly calves (as is the case on this report) and young adults submitted to environmental or management-related stressors, including weaning, high concentration of cattle, transportation, and introduction of new animals into the herd.6-7 The stressors conditions precipitating the disease in the present case were not identified. However, the calf has been transported some months before the onset of clinical disease, which most likely provided close contact with other animals and might have facilitated infection or reactivation of a latent herpesviral infection.3,4 BoHV-1 and BoHV-5 are genetically and antigenically related viruses that belong to the family Herpesviridae, subfamily Alpha-herpesvirinae, genus Varicellovirus.2 BoHV-1 has been historically associated with abortion, respiratory, and genital disease, namely infectious bovine rhino-tracheitis and infectious pustular vulvovaginitis or balanoposthitis. Although BoHV-5 has been classically associated with neurological disease in cattle, it is currently believed that both BoHV-1 and BoHV-5 cause identical neurological disease in endemic areas and cases of meningoencephalitis caused by BoHV-1 are not as uncommon as previously suspected.7,9
Necrotizing meningoencephalitis due to BoHV has been described worldwide, particularly in South America.4,5,9 The reason for the lower incidence of meningoencephalitis due to BoHV in other parts of the world is unknown, but it has been proposed that widespread vaccination against BoHV in North America and Europe protects susceptible animals and prevents clinical disease in these areas.10 Viral transmission occurs via direct or indirect contact among susceptible individuals, with primary viral replication occurring in the ocular and oropharyngeal mucosal epithelium. Following primary replication, viral particles reach the rostral portions of the brain and sensory ganglia via axonal retrograde transportation and direct invasion through the olfactory bulb and trigeminal nerves.2
Viral invasion into the brain may result in secondary replication and neurological disease or subclinical infection and viral latency in the trigeminal ganglia and central nervous system.3 Latently infected individuals will become an important source of virus to other susceptible cattle in the case of virus reactivation.3,4 A hemato-genous route of infection has been proposed, but it seems less likely due to the characteristic distribution of the lesions in the frontal areas of the brain, which support direct viral invasion through the olfactory bulb.3,7 The gross and microscopic findings reported in this calf are typical of those observed in cases of meningoencephalitis due to either BoHV-1 or BoHV-5. In addition, cases of neurological disease by BoHV with neither gross nor microscopic lesions despite the development of severe neurological disease have been observed.7,8 These variations in the pathological presentation of neurological disease due to BoHV are attributed to potential differences in the neurovirulence or to individual susceptibility of animals to viral infection.1,8
JPC Diagnosis:
Conference Comment:
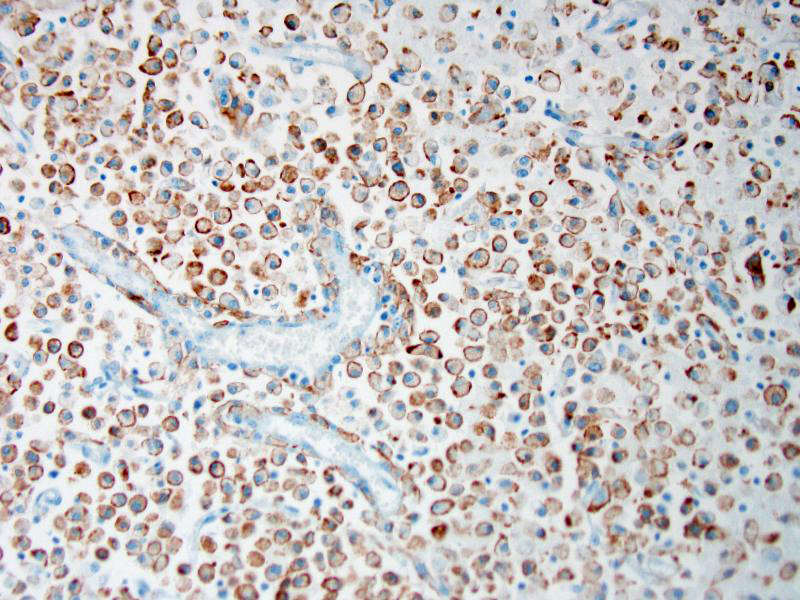
Attendees also described numerous microglial phagocytic cells with an abundant amount of foamy eosinophilic cytoplasm (gitter cells) infiltrating into the necrotic areas. Gitter cells are microglial cells that transformed into phagocytic macrophages within the central nervous system and function to engulf cellular debris and degenerate myelin.2 Microglial phagocytes also surround degenerate neurons (satellitosis) and phagocytose degenerate neuronal cell bodies (neuronophagia) during neurotropic viral infection. In severe lesions, as in this case, gitter cells can arise from peripheral blood monocytes as well as microglial cells.2 These cells are nicely highlighted by intense membranous and cytoplasmic immunoreactivity for ionized calcium binding adapter molecule 1 (IBA-1), an immunohistochemical stain commonly used to identify microglial cells and macrophages run by the Joint Pathology Center prior to the conference.
Conference participants discussed differential diagnoses for laminar cortical necrosis and polioencephalomalacia in cattle. These include sulfur intoxication, sodium chloride intoxication/water depri-vation, lead intoxication, and thiamine deficiency. Participants also discussed other infectious diseases that can cause neurologic disease in cattle, such as rabies virus, malignant catarrhal fever (MCF), listeriosis, and the neurotropic amoeba, Naegleria fowleri.2,8,9,15 The conference moderator noted that this case has excellent examples of ferruginated neurons. These are deeply basophilic dead neurons that become encrusted and replaced with finely beaded material interpreted as mineral, rather than being removed by phagocytosis. The mineral is composed of both calcium and iron and affected neurons are highlighted by both von Kossa and Perls Prussian blue staining techniques. These neurons are reported to occur in areas of ischemic and hypoxic damage in the central nervous system, particularly in juveniles and neonates.5
References: