Signalment:
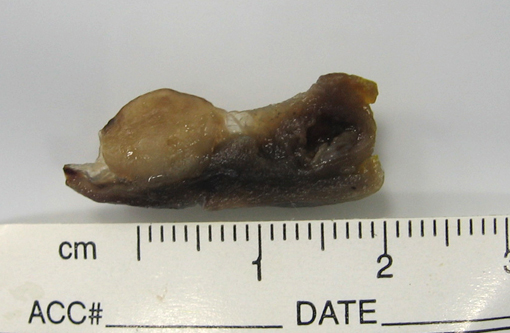
Gross Description:
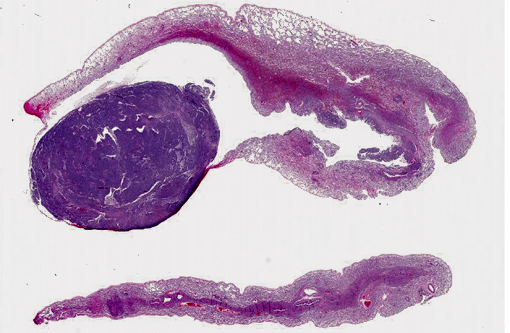
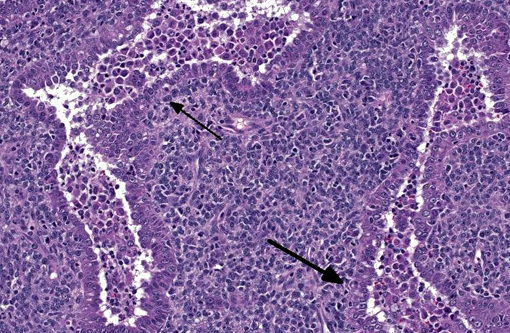
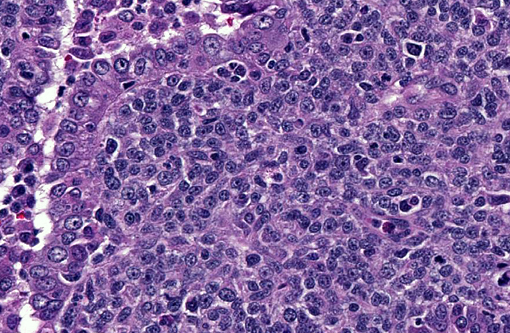
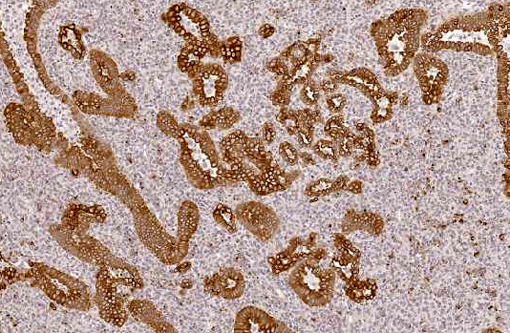
Histopathologic Description:
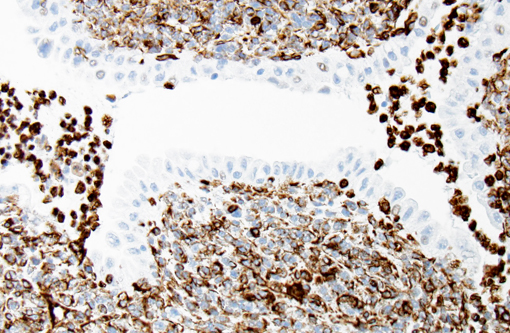
With immunohistochemistry, the adenocarcinomatous component of the tumor was strongly positive for pancytokeratin markers AE1 and AE3. Although pancytokeratin expression was negative for the vast majority of the small cell component, scattered individual cells displayed strong cytoplasmic expression for both markers (Fig. 2 and 3). Neither the adenocarcinomatous or small cell component expressed synaptophysin, chromogranin or neuron specific enolase (NSE). Small numbers of scattered cells throughout the tumors expressed CD20 and CD18 and were interpreted in combination with the H&E section as leukocytes (secondary inflammation). Histopathologic examination of the left mandibular mass (not provided) was consistent with a salivary gland adenocarcinoma. Sections from the left adrenal gland (not provided) revealed a malignant pheochromocytoma. The hepatic nodule from the right medial lobe (not provided) displayed the same morphology as observed in the pulmonary nodule and was interpreted as a focus of metastasis from the pulmonary neoplasm.
Morphologic Diagnosis:
Condition:
Contributor Comment:
Combined tumors are neoplasms that display more than one cellular morphology and microscopic architecture, often composed of cells with remarkably different histological, histochemical and immunohistochemical properties.(8) One example of combined tumor in humans is combined pulmonary carcinomas, which have been reported to exhibit combinations of up to 4 distinct morphologies (squamous cell carcinoma, small-cell/neuroendocrine carcinoma, blastoma and adenocarcinoma).(9) Additionally, extra-pulmonary combined tumors with a small cell/ neuroendocrine component associated with a component of squamous cell carcinoma or adenocarcinoma appear to be relatively common in the rare group of small cell carcinomas in people, and have been reported in the esophagus, prostate gland and colon.(10) Aside from combined pulmonary carcinoma of dogs and cats, other examples of combined tumors in domestic animals include carcinosarcomas of mammary gland and combined hepatocellular-cholangiocellular carcinoma.(13)
Overall, primary pulmonary neoplasms are exceedingly common in people and uncommon in dogs.(1) The prevalence of primary lung cancer in the overall canine population has been reported to be of 1% of all diagnosed canine neoplasia.(1,3,12) The most accepted veterinary classification of pulmonary neoplasms to date includes adenomas and papillomas, bronchial gland carcinoma, squamous cell carcinoma, adenocarcinomas of papillary, acinar, solid and mixed types, bronchioloalveolar carcinoma, adenosquamous carcinoma, small and large cell carcinomas, neuroendocrine carcinoma, pulmonary blastoma and combined carcinoma.(4,14) Adenocarcinomas appear to be the most common type of lung cancer in domestic animals.(3) In contrast to the veterinary classification, in people, combined carcinoma is classified as a subvariant of small cell carcinomas, since it has been shown that the small cell component in those tumors has neuroendrocrine properties. The importance of proper tumor classification in this instance lies in the fact that prognostic outcome and therapeutic management largely depend on the leading histomorphological tumor type.(6) The same has not been proven in the rare cases of combined pulmonary tumors of domestic animals(4), where the small cell component has failed to express the most common neuroendocrine immunohistochemical markers, such as chromogranin, synaptophysin and neuron-specific enolase (as in the present case), and accurate definition of histogenesis of this component remains unavailable. To date, the histogenesis of the small cell component of combined pulmonary tumors of domestic animals has only been morphologically and immunohistochemically characterized as a pulmonary basaloid cell. The difficulty in further analyzing these tumors at the molecular level in domestic animals is most likely due to the rarity of the tumor, which has only been reported in cats and less commonly dogs.(4)
Despite the high prevalence of lung cancer in people, combined pulmonary tumors are also rare in this population. In a recent retrospective study of 1,158 pulmonary surgical specimens of people, combined carcinomas represented 1.8% of all pulmonary tumors.(5) Nevertheless, a few studies in human specimens have been able to provide further insight on what seems to be the most intriguing and fascinating aspect of this rare neoplastic entity; the nature of its capability to produce such phenotypically distinct components. The main questions raised around this entity are whether the different components of the tumors are genetically related and why and how differentiation towards such different cellular components occurs.(5,8,9,11) Answer to these questions may shed light on the histogenesis and biologic behavior of the tumor cells. Clonality assays and study of LOH (loss of heterozygosity) have been employed to aid in answering these questions; however, so far, the results raise more questions than definitive results.
To date, a few studies have shown monoclonality and identical genetic alterations in the specific chromosomes analyzed in a few of these tumors, raising the hypothesis that the different cellular components of the tumors are likely derived from a common precursor cell and driven by the same carcinogens.(5,8,9,11) In these cases, it remains to be understood why cells with a common genetic profile display such distinct morphology, histochemical and immunohistochemical properties. It can be hypothesized that during malignant transformation, some of the progeny cells derived from a common precursor cell may convert to a different morphology under influence of different environmental stimuli. Alternatively, the clonal neoplastic cell may retain the ability to differentiate toward other morphologic phenotypes in response to different stimuli.(8)
Contrary to those results, one study has shown a few combined tumors with different genetic profiles, despite such close morphologic relationship of the different cellular components. In those cases, it was hypothesized that the different groups of cells (small cell component with squamous cell differentiation and a separate adenocarcinomatous component) may have undergone separate progression from a pluripotent single clone in a very early stage, resulting in distinct genetic abnormalities that may have developed in a later phase of the tumor progression.(11) Further clonality and LOH studies on this rare neoplastic entity are still necessary and would help to provide additional insight in the field of stem cells, tumor biology and oncologic pathology. The concomitant development of multiple different neoplasms in this dog (salivary gland adenocarcinoma, combined pulmonary carcinoma and pheochromocytoma) is also an interesting point of discussion beyond the scope of this brief review.
JPC Diagnosis:
Conference Comment:
As previously outlined, the reactivity of cytokeratin within both cell populations is characteristic to this diagnosis. The conference discussion, however, was focused on the finding of diffuse immunoreactivity among the small cell population with vimentin leading some to consider the diagnosis of carcinosarcoma for this case. Carcinosarcomas usually have areas of osseous or chondrous metaplasia within the mesenchymal cell population4; and the small cell component in this case does not have spindle cell morphology, thus our agreement with the contributors diagnosis.
Participants noted the primitive morphology and loss of polarity within the small cell component. This led to a discussion on the epithelial-mesenchymal transition (EMT) as being a possible cause for the change in cellular morphology as well as the expression of vimentin, which would be consistent with the presence of metastasis observed in this case.
The EMT is a well-defined process of transformation during which neoplastic cells acquire the ability to invade and disseminate. Included in this transformation is a conversion from a polygonal to spindle morphology along with the repression of E-cadherin expression.(7) Loss of E-cadherin, a key cellular adhesion molecule, eliminates the cellular restraint mechanism within the tissue of origin thereby permitting dissemination. This transformation has been characterized histologically by increased expression of vimentin, which also has been found to correlate with grading criteria in some neoplasms.(15) While there have been studies examining cytokeratin and vimentin expression in canine pulmonary tumors, a correlation with histologic grade has not been demonstrated.(2)
References:
1. Buendia AJ, Sanchez A, Martinez CM, Navarro JA. Immunohistochemical characterization of a pulmonary large-cell carcinoma in a dog. Vet Pathol. 2008;45:484-485.
2. Burgess HJ, Kerr ME. Cytokeratin and vimentin co-expression in 21 canine pulmonary epithelial neoplasms. J Vet Diagn. 2009;21:815-820.
3. Castellano MC, Massonez AR, Idiart JR. Primary pulmonary adenocarcinoma metastatic to the uvea, brain, and adrenal gland in a dog. J Vet Med A. 2006;53:194-197.
4. Dungworth DL, Hauser B, Hahn FF, Wilson DW, Haenichen T, Harkema JR. Histological Classification of Tumors of the Respiratory System of Domestic Animals. 2nd ed. Vol VI. Washington, DC: Armed Forces Institute of Pathology, American Registry of Pathology. 1999.
5. Fellegara G, Dadda T, Pilato FP, Froio E, Ampollini L, Rusca M, et al. Genetics of a combined lung small cell carcinoma and large cell neuroendocrine carcinoma with adenocarcinoma. Virchows Arch. 2008;453:107-115.
6. Hahn FF, Muggenburg BA, Griffith WC. Primary lung neoplasia in a Beagle colony. Vet Pathol. 1996;33:633-638.
7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674.
8. Huang J, Behrens C, Wistuba II, Gazdar AF, Jagirdar J. Clonality of combined tumors. Arch Pathol Lab Med. 2002;126:437-441.
9. Iezumi K, Masugana A, Kadofuku T, Iwamoto S, Masuda M, Suzuki S, et al. Combined small cell carcinoma with pulmonary blastoma and adenocarcinoma. Case report and clonality analysis. Pathol Res Pract. 2006;202:895-899.
10. Junker K, Wiethege T, Muller KM. Pathology of small-cell lung cancer. J Cancer Res Clin Oncol. 2000;126:361-368.
11. Murase T, Takino H, Shimizu S, Inagaki H, Tateyama H, Takahashi E, et al. Clonality analysis of different histological components in combined small cell and non-small cell carcinoma of the lung. Hum Pathol. 2003;34(11):1178-1184.
12. Polton GA, Brearley MJ, Powell SM, Burton CA. Impact of primary tumor stage on survival in dogs with solitary lung tumors. J Small An Pract. 2008;49:66-71.
13. Shiga A, Shirota K, Enomoto M. Combined hepatocellular and cholangiocellular carcinoma in a dog. J Vet Med Sci. 2001;63(4):483-486.
14. Wilson DW, Dungworth DL. Tumors of the respiratory tract. In: Meuten DJ, ed. Tumors in Domestic Animals. 4th ed. Ames, IA: Iowa State Press; 2002:365-399.
15. Zhao Y, Yan Q, Long X, Chen X, Wang Y. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem Funct. 2008;26:571-577.