Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 7
9 October 2019
Dr. John Cullen, VMD,
PhD, DACVP, FIAT
Professor, Anatomic Pathology
College of Veterinary Medicine
North Carolina State University
Raleigh, North Carolina
CASE IV: 19-117 (JPC 4134512).
Signalment: 15-year-old, castrated male, Quarter Horse (Equus caballus)
History: This horse presented to ambulatory service with an acute onset of illness. Clinical examination revealed a normal respiratory rate, normal heart rate and a temperature of 100.7 oF. There was severe icterus and dark brown urine dripped from the penis. Gut sounds were decreased in all 4 quadrants. When moved, the horse collapsed and euthanasia was elected. The horse was not vaccinated. There was no history of feed change, medications, travel or exposure to toxins.
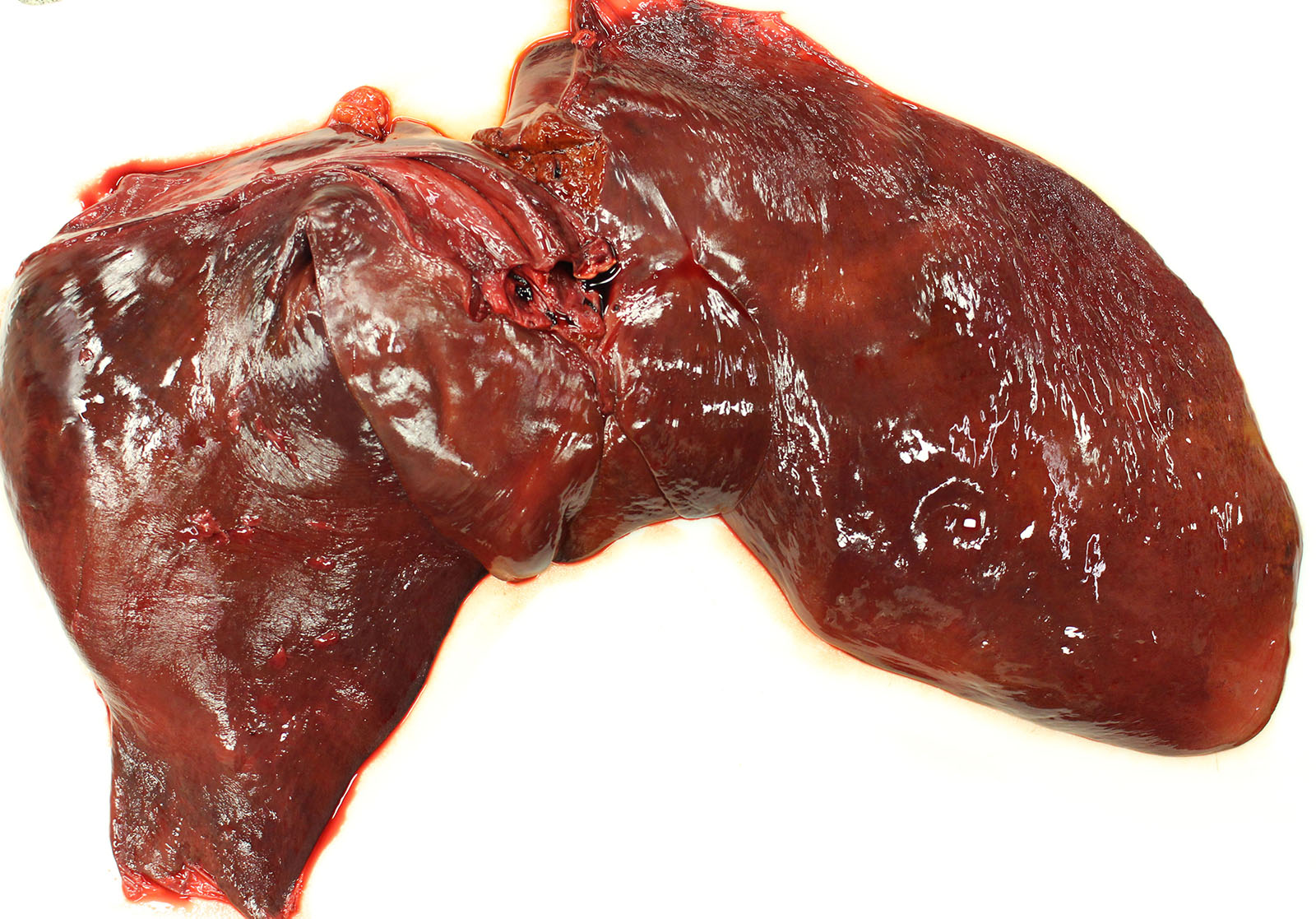
Gross Pathology: Gross examination revealed a carcass in good nutritional condition with no evidence of dehydration and moderate autolysis. Dark red urine dripped from the prepuce. There was diffuse and severe icterus. A scant amount of serosanguinous fluid was noted in the peritoneum. The liver was small and flaccid weighing 3.8 kg or 0.7% body weight (normal range 1.2-1.5%). On section, an enhanced reticular pattern was noted. The small intestinal content was hemorrhagic. The urinary bladder was filled with dark red urine and the discoloration did not clear following centrifugation (pigmenturia). There was diffuse splenic congestion consistent with barbiturate euthanasia.
Laboratory results: PCR was positive for equine parvovirus (EqPV-H). PCR was negative for equine pegivirus (EPgV), non-primate hepacivirus (NPHV) or equine hepacivirus and Theiler’s disease-associated virus (TDAV). PCR was negative for Leptospira species.
Urine sediment: 11-20 ammonium biurate crystals /hpf
Microscopic Description:
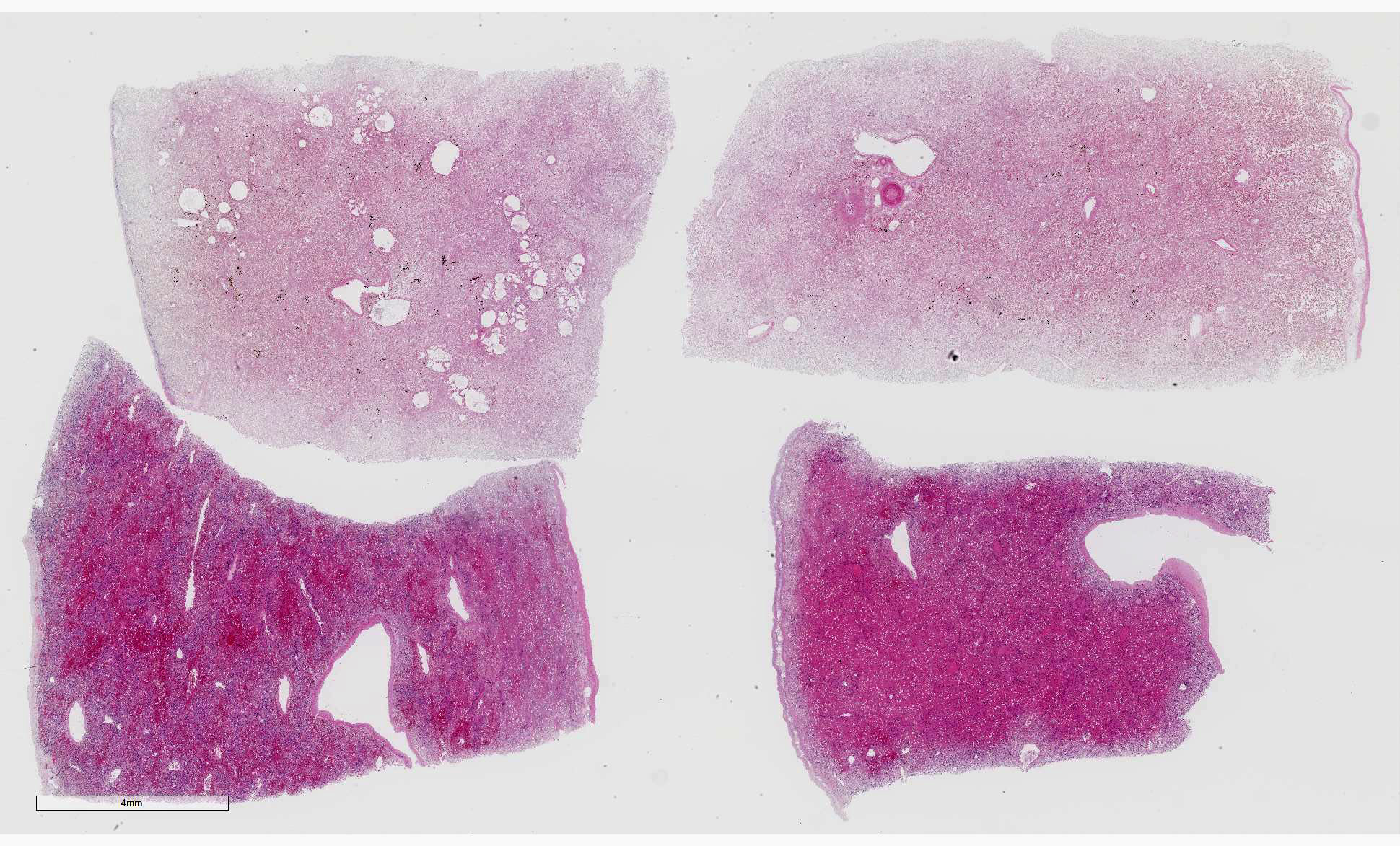
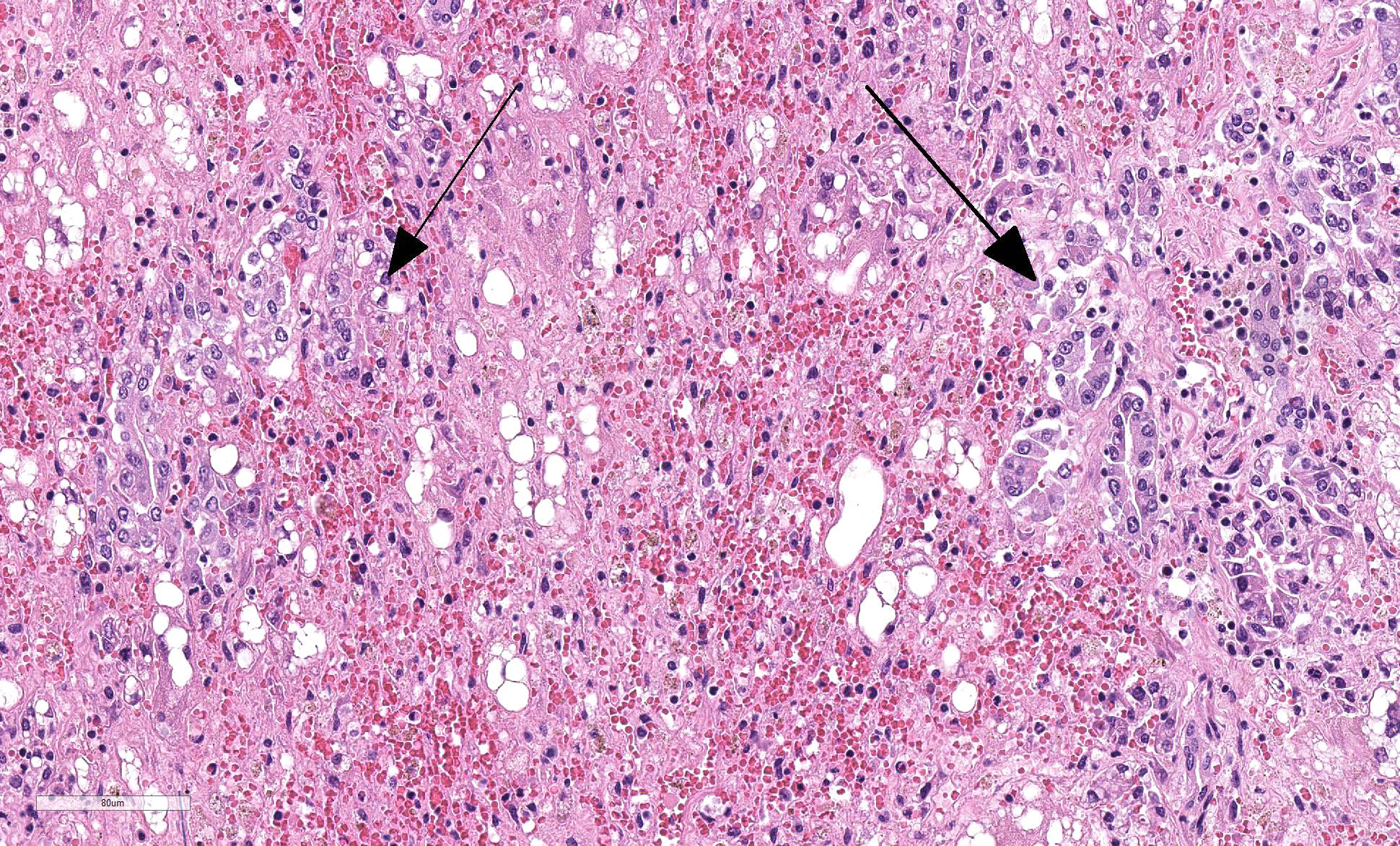
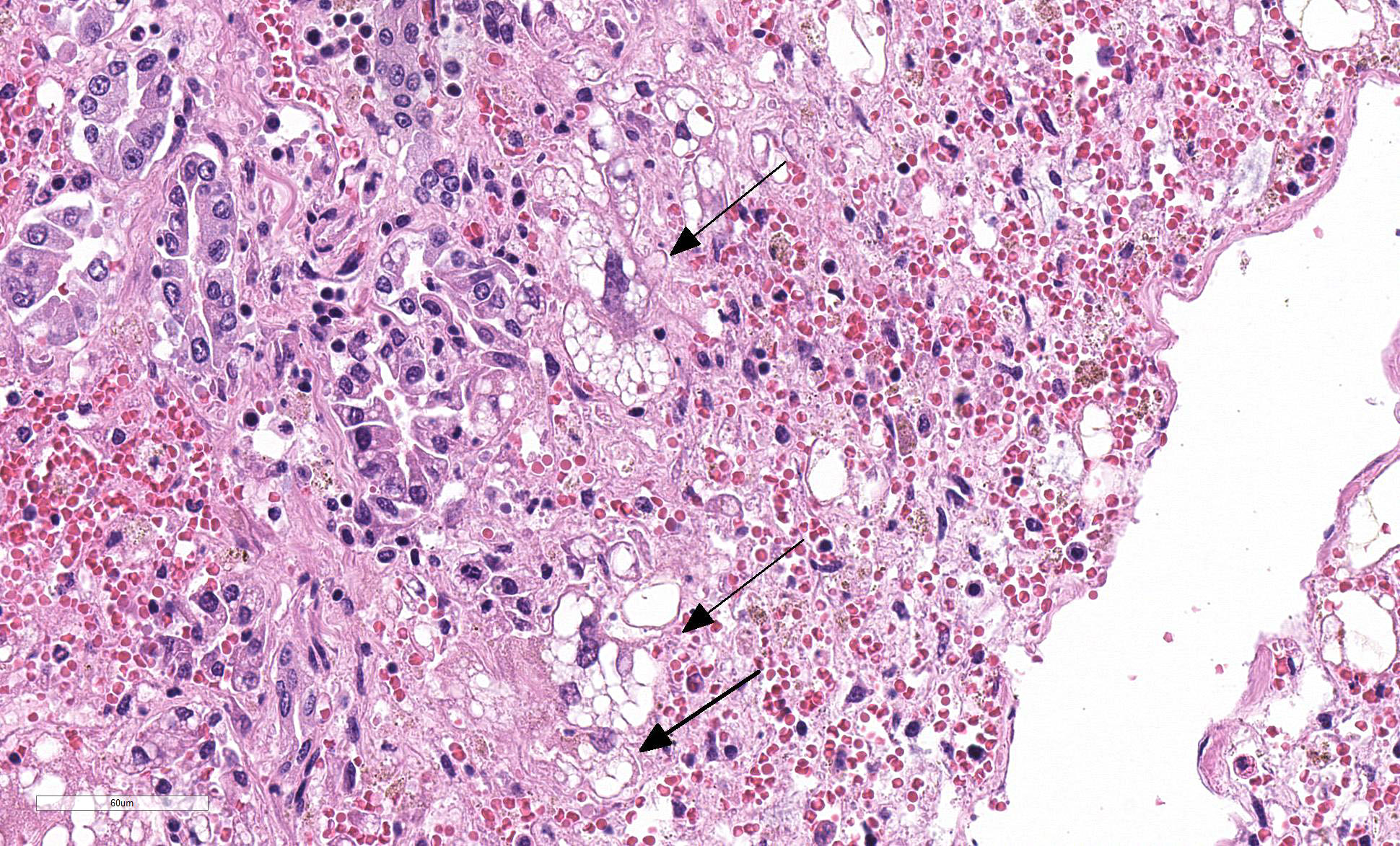
Liver: Four sections are available for examination with varying degrees of autolysis and postmortem bacterial overgrowth. Diffusely, the hepatic lobules are distorted and reduced in size owing to marked loss of centrilobular and midzonal hepatocytes. This is accompanied by stromal collapse and sinusoidal congestion. Individualized periportal hepatocytes remain along the periphery of the lobules. Periportal hepatocytes have abundant cytoplasm containing multiple, large, well-defined vacuoles consistent with macrovesicular fatty change. Large, multinucleated hepatocytes are frequently observed (hepatocellular giant cell or syncytial cell formation). Kupffer cells contain a moderate amount of light brown, granular pigment. The portal units are expanded by minimal fibrosis, infrequent duplication of the bile ducts and a scant infiltrate of mononuclear inflammatory cells including lymphocytes, histiocytes and plasma cells. Copper was not observed on rhodanine stain and a scant amount of iron restricted to the Kupffer cells was noted on Perls’ Prussian blue stain.
Kidney (not shown on slide): Multifocally renal tubules are ectatic, are lined by attenuated epithelium and contain granular to hyaline, bright orange to red material interpreted to be heme pigment.
Contributor Morphologic Diagnosis:
Liver: Hepatocellular loss and degeneration, submassive, severe, subacute with stromal collapse, mild portal hepatitis and syncytial cell (giant cell) formation
Contributor Comment: Based on the clinical course of disease, gross pathology, histopathology, PCR results and lack of exposure to known hepatotoxins, this is interpreted to be a case of equine serum hepatitis or Theiler’s disease. Serum hepatitis is a long recognized cause of acute liver failure in horses. Theiler’s disease was first described in 1919 in horses following vaccination using equine antiserum against African horse sickness.4,13 The disease is reported globally and is typically observed following administration of equine biological products including tetanus antitoxin, botulinum antitoxin, antiserum against Streptococcus equi, pregnant mare’s serum and equine plasma.5,14 Of these products, serum hepatitis is most commonly associated with tetanus antitoxin possibly because it is the most frequently used.14 Similar to the current case, a number of cases of equine serum hepatitis are not associated with administration of equine biologicals raising the specter of horizontal transmission from contaminated medical equipment and insect vectors such as tabanid flies. 2,3,15
The typical incubation period is 4-10 weeks, but can be as long as 14 weeks.4,5,14 The onset of clinical disease is sudden with rapid progression to death. Lethargy, icterus, photosensitivity, fever and neurological signs including hyperexcitability, blindness and ataxia are reported.4,13 Morbidity rates vary from 1-18% in outbreaks and mortality rates are between 50-90%.14
Typical biochemical findings include marked elevation in liver enzyme values (primarily SHD and AST and to a lesser degree GGT), direct and indirect bilirubin and bile acids.5,14 Reported gross lesions include icterus, ascites, serosal and renal petechiae, and intestinal hemorrhage. The liver is small and limp due to the hepatocellular loss and is often described as having a "dishrag" appearance. Cut surface reveals a highlighted zonal pattern.2, 4 Acute hepatic necrosis and hemorrhage are not features of serum hepatitis. Microscopic features are instead more chronic than the clinical course suggests and are dominated by hepatocellular loss with collapse and distortion of the reticulin framework as seen in the current case. There may be few surviving periportal hepatocytes which demonstrate hepatocellular vacuolation consistent with macrovesicular lipidosis. Additional microscopic features include variable numbers of apoptotic hepatocytes, deposition of bile pigments in Kupffer cells and hepatocytes, mild fibroplasia in the portal units and variable ductular proliferation. Inflammation is not robust, but a diffuse infiltrate of lymphocytes, plasma cells, histiocytes and neutrophils may be present.2, 4
While the association between serum hepatitis and administration of equine biologics has been known for 100 years and a blood-borne virus has long been suspected, the etiologic agent of Theiler’s disease has eluded the veterinary profession. Since 2012, four emerging viruses have been identified and studied for their possible association with equine hepatitis. Three are in the family Flaviviridae including equine pegivirus (EPgV), non-primate hepacivirus (NPHV) or equine hepacivirus, and Theiler’s disease-associated virus (TDAV). The fourth is equine parvovirus-hepatitis (EqPV-H). NPHV, closely related to hepatitis C virus, is hepatotropic and hepatic disease following experimental transmission is documented.9 TDAV was first identified in an outbreak of acute clinical hepatitis in horses following administration of botulinum antitoxin of equine origin.3 However, retrospective analysis of samples indicated the presence of EqPV-H in all TDAV positive samples indicating coinfection initially missed do to methodology.5 EPgV is a common infection of horses in the United States and Western Europe, but the virus is not hepatotropic and has not been associated with hepatic disease.6 Recently, EPgV infection was associated with a reduced risk of having increased liver enzyme activity indicating that EPgV is an unlikely cause of equine hepatitis.10 In 2018, a novel equine parvovirus (equine parvovirus-hepatitis, EqPV-H) was discovered in the liver and serum of a horse succumbing to Theiler’s disease and experimental administration of EqPV-H positive tetanus antitoxin samples resulted in viremia and subclinical or clinical hepatic disease in 2 horses. This study also revealed EqPV-H viremia in 13% of normal horses tested suggesting that many infected horses dont develop clinical disease.5 More recently, 2 companion studies demonstrated EqPV-H infection in 18 consecutive cases of serum hepatitis and EqPV-H infection in 9/10 horses with Theiler’s disease in the absence of equine biologic product administration. In both studies, the 3 additional flaviviruses implicated in equine hepatitis were absent or rarely present.14,15 These recent studies support the role of EqPV-H in Theiler’s disease, but further studies are required to unequivocally demonstrate that EqPV-H is the cause of serum hepatitis.
In the standard veterinary pathology textbooks, giant cell formation is not described as a feature in equine serum hepatitis and was an unexpected feature in this case. There is a single report of Theiler’s disease in a Canadian horse in which multinucleated hepatocytes are described.13 Giant-cell hepatitis (GCH) is well-described in humans and is characterized by parenchymal inflammation with formation of large multinucleated hepatocytes.11 It is most commonly seen in neonates, and occurs rarely in adults where it is known as post-infantile GCH.11,16 It is considered to be a nonspecific tissue reaction to various stimuli and therefore is not specific to an etiology. The pathogenesis is unknown and may be due to hepatocyte nuclear proliferation without subsequent cell division or membrane fusion of neighboring hepatocytes.11 In infants, a recent study indicated that most cases of GCH were idiopathic. In this case series, panhypopituitarism was the most common recognizable clinical association with fewer cases attributed to biliary atresia, Alagille syndrome, bile salt defects, neonatal hemochromatosis, SCID, and viral infections.16 In adults, giant cell transformation occurs with a variety of insults including viral hepatitis (hepatitis A, hepatitis B, non A, non B hepatitis, hepatitis C, and Epstein-Barr virus), autoimmune hepatitis, and with a variety of drugs and herbal remedies.11 Giant cell hepatitis is an uncommon lesion in animals but has been recorded in cats, calves and foals.4 In foals, giant cell transformation has been reported in cases of neonatal isoerythrolysis1 and in aborted foals with suspected leptospirosis.17
In the absence of muscle injury in this horse, the dark red appearance to the urine and pigmentary nephrosis were interpreted as hemoglobinuria and hemoglobinuric nephrosis indicative of intravascular hemolysis. Common causes of intravascular hemolysis and hemoglobinuria in horses include immune-mediated hemolytic anemia; a number of infections including piroplasmosis, equine infectious anemia, leptospirosis, and clostridial hepatitis; drug toxicity; toxic plants including red maple, Brassica species and membranes of the onion family; and hepatic failure. Hemolysis secondary to hepatic failure is presumed in this case and intravascular hemolysis is described in the terminal stages of Theiler’s disease.2 The mechanism for intravascular hemolysis in cases of liver failure in horses is unknown; however, bile acids or their salts are considered possible hemolytic factors.8
Contributing Institution:
Diagnostic Services Unit
University of Calgary, Faculty of Veterinary Medicine
JPC Diagnosis: Liver: Degeneration, necrosis and loss, massive, diffuse, severe, with stromal collapse and hepatocellular lipidosis.
JPC Comment: The contributor has done an outstanding job summarizing the history, pathogenesis, and current thought regarding this very dramatic and well-known disease of the horse.
Sir Arnold Theiler 1867-1936), for which this disease still bears his name, was a Swiss veterinarian and researcher of great importance in the area of veterinary research and education in South Africa. As the state veterinarian for the South African Republic, he developed he first vaccine against rinderpest. In 1919, he was the first to describe acute serum hepatitis in animals vaccinated against African horse sickness. As the first director of the Onderstepoort veterinary Research Institute, he was instrumental in leading the research team in their investigations of many diseases of the region including East Coast fever (caused by Theileria parva), sleeping sickness, heartwater, malaria, and African Horse sickness. He shortly became the dean of the newly built University of Pretoria Faculty of Veterinary Science, and worked there until his passing.
Since the identification of an equine parvovirus as the putative agent of equine serum hepatitis, a number of groups have begun the process of determining the extent of its presence in a variety of equine biologics, including horse serum from a variety of providers. In one study,7 11 out of 18 samples of serum from providers in the US, Europe, Canada, New Zealand and South America demonstrated the presence for equine parvovirus-hepatitis (EqPV-H) using gel electrophoresis of the qPCR products and anti-EQPv-H antibodies were also detected in the same samples, although both fetal-based serum products tested negatively. While the presence of the putative agent has only been definitively identified in conjunction with disease in the US and China at this time, this study shows that the putative agent is truly of global origin.7
One of the classic syndromes associated with equine serum hepatitis and the reason it has longed been considered an infectious disease rather than just a reaction to biologics is the propensity for horses in contact to develop the disease. A 2018 study15 reports the results of identification of EPv-H from 10 cases of apparent serum hepatitis on 6 different properties and the results of screening of in-contact horses which had not previously received any equine biologicals. In this study, animals with clinical infection were 80% positive, while in-contact animals were 48% positive, suggesting that these animal may be subclinical infected. Samples from infected animals were also tested for other viruses that had previously been associated with equine serum hepatitis (non-primate hepacivirus A), EPgV (pegivirus E) and EDAV (pegivirus D) were not identified in this study. 15
Due to the massive degeneration, necrosis and hemorrhage
affecting much of the four sections on the slide, this proved to be a
deceptively difficult slide to describe. A common artifact was present in many
of the slides which appears as blue spicular crystals within vacuoles of fat
which was interpreted as calcium stearate crystals.
References:
1. Boyle AG, Magdesian KG, Ruby RE. Neonatal isoerythrolysis in horse foals and a mule foal: 18 cases (1988-2003). J Am Vet Med Assoc. 2005; 227(8): 1276-1283.
2. Brown DL, Van Wettere AJ, Cullen JM. Hepatobiliary system and exocrine pancreas. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, USA: Elsevier; 2017: 412-470.
3. Chandriani S, Skewes-Cox P, Zhong W, et al. Identification of a previously undescribed divergent virus from Flaviviridae family in an outbreak of serum hepatitis. Proc Natl Acad Sci USA. 2013; 110(15): E1407-E1415.
4. Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmerâs Pathology of Domestic Animals. 6th ed. St. Louis USA: Elsevier; 2016: 259-351.
5. Divers TJ, Tennant BC, Kumar A, et al. New parvovirus associated with serum hepatitis in horses after inoculation of common biological product. Emerg Infect Dis. 2018; 24(2): 303-310.
6. Kapoor A, Simmonds P, Cullen JM, et al. Identification of a pegivirus (GB virus-like virus) that infects horses. J Virol. 2013; 87(12): 7185-7190.
7. Meister TL, Tegtmeyer B, Postel A, Cavalleri JMV, Todt D, Stang A, Steinmann E. Equine parvovirus-hepatitis frequently detectable in commercial equine serum pools. Viruses 2019; 11(5):E461.
8. Ramaiah SK, Harvey JW, Giguere S, Franklin RP, Crawford PC. Intravascular hemolysis associated with liver disease in a horse with marked neutrophil hypersegmentation. J Vet Intern Med. 2003; 17:360-363.
9. Ramsay JD, Evanoff R, Wilkinson TE, et al. Experimental transmission of equine hepacivirus in horses as a model for hepatitis C virus. Hepatology. 2015; 61(5): 1533-1546.
10. Ramsay JD, Evanoff R, Mealey RH, Simpson EL. The prevalence of elevated gamma-glutamyltransferase and sorbitol dehydrogenase activity in racing Thoroughbreds and their association with viral infection. Equine Vet J. 2019 Mar 8. doi:10.1111/evj.13092.
11. Shetty S, Janarthanan K, Leelakrishnan V, Nirmala V. Giant-cell hepatitis-rare entity in adults. J Clin Exp Hepatol. 2016; 6(3): 244-245.
12. Smith HL, Chalmers GA, Wedel R. Acute hepatic failure (Theiler’s disease) in a horse. Can Vet J. 1991; 32: 362-364.
13. Sturgeon B. Theiler’s disease. Vet Rec. 2017; 180(1):14-15.
14. Tomlinson JE, Kapoor A, Kumar A, et al. Viral testing of 18 consecutive cases of equine serum hepatitis: a prospective study (2014-2018). J Vet Int Med. 2019; 33: 251-257.
15. Tomlinson JE, Tennant BC, Struzyna A, et al. Viral testing of 10 cases of Theiler’s disease and 37 in-contact horses in the absence of equine biologic product administration: a prospective study (2014-2018). J Vet Int Med. 2019; 33: 258-265.
16. Torbenson M, Hart J, Westerhoff M, et al. Neonatal giant cell hepatitis: histological and etiological findings. Am J Surg Pathol. 2010; 34(10): 1498-1503.
17. Wilkie IW, Precott JF, Hazlett MJ, Maxie MG, van Dreumel AA. Giant cell hepatitis in four aborted foals: a possible leptospiral infection. Can Vet J. 1988; 29:1003-1004.