Wednesday Slide Conference, 2023-2024, Conference 5, Case 3
Signalment:
11-year-old, male intact common marmoset, primate (Callithrix jacchus)
History:
This male marmoset’s clinical history included significant weight loss, poor body condition, chronic intermittent diarrhea, and progressive reticulocytosis, thrombocytosis, and hypoalbuminemia. Diagnostic evaluation ruled out infection by Giardia spp., Klebsiella pneumoniae, Campylobacter coli, C. jejuni, Salmonella spp., and Shigella spp., and revealed two biotypes of E. coli on fecal culture. Late in the disease course, there was radiographic evidence of an expansile lesion of the left patella. Treatment with nutritional support, antimicrobials, and immunomodulatory medications yielded minimal and transient improvement.
Gross Pathology:
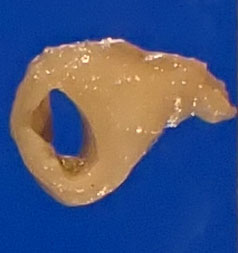
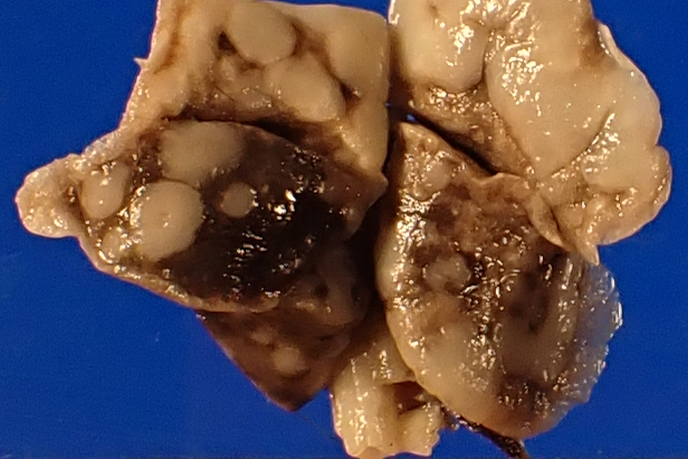
Gross postmortem evaluation demonstrated little to no subcutaneous, visceral, or perirenal adipose tissue. Within all lung lobes were innumerable coalescing, firm, tan nodules, measuring up to 4 mm in diameter. The small intestine from the level of the pylorus and extending distally 8 cm was paler than the more normal, aborad, intestine. There was transmural thickening of the proximal duodenum, measuring up to 7 mm on cross section.
Laboratory Results:
Significant clinical pathology values: thrombocytosis (1,042 k/μL) and hypoalbuminemia (2.9 g/dL).
Microscopic Description:
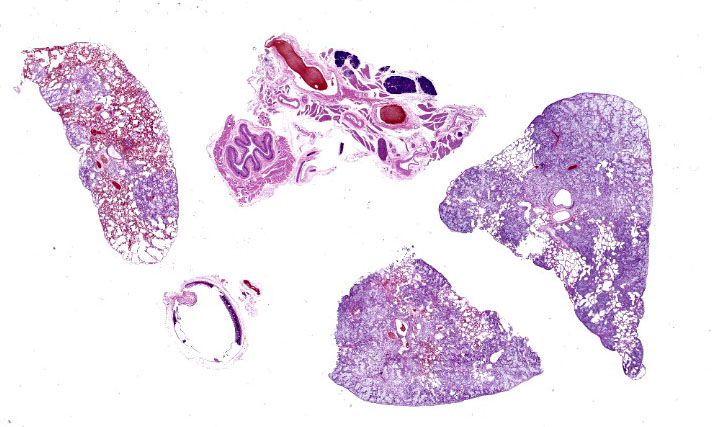
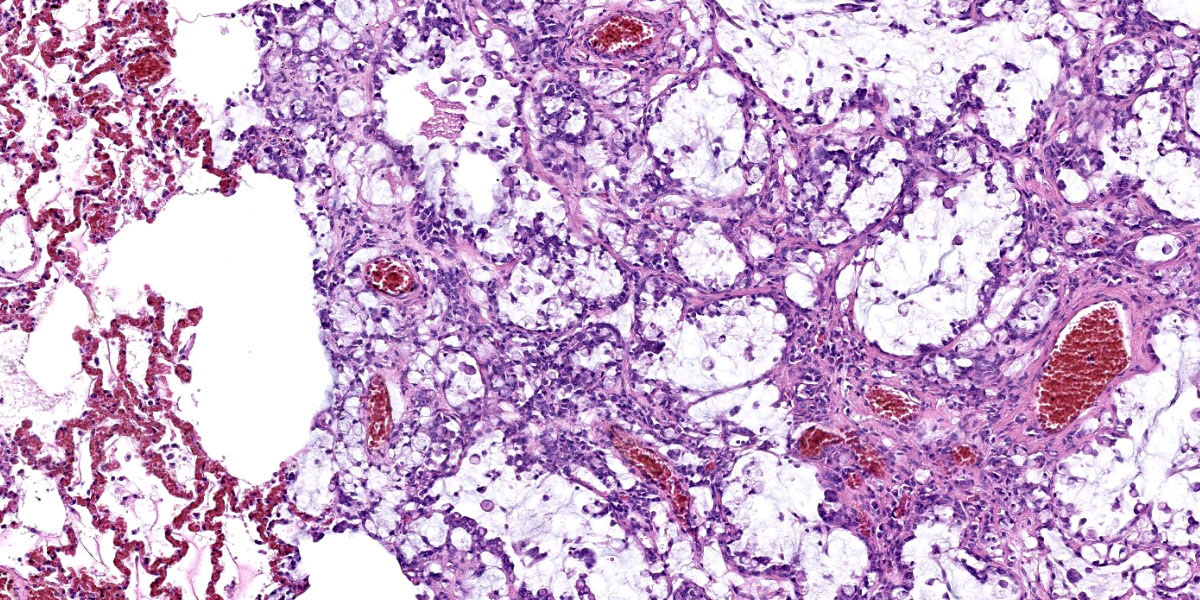
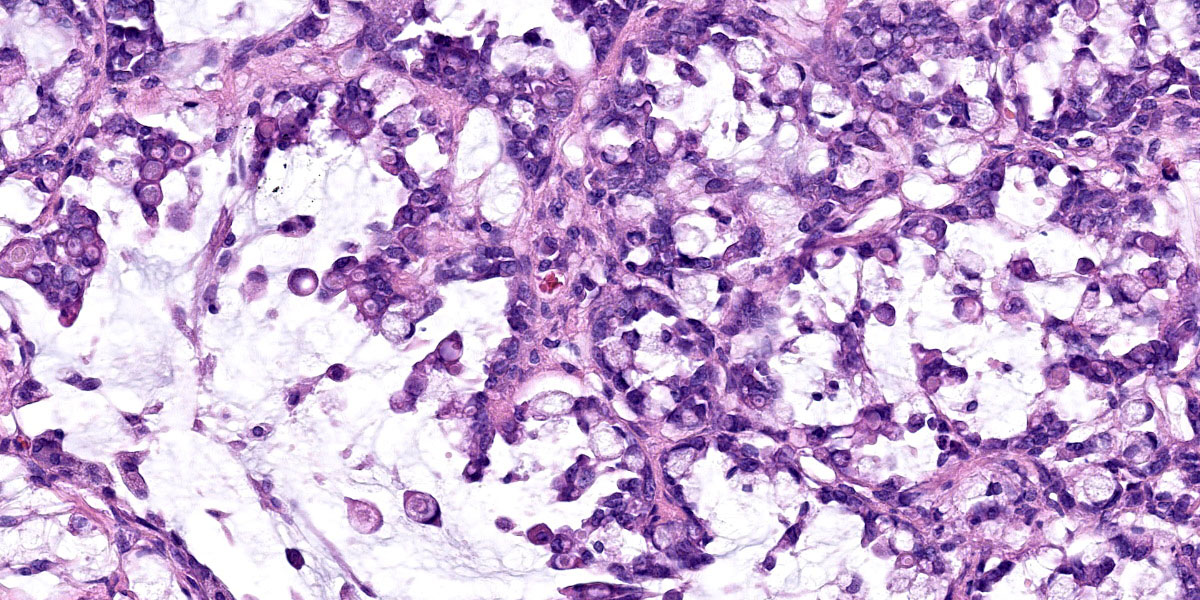
Effacing and replacing approximately 75-80% of the pulmonary parenchyma are multiple neoplastic masses that are moderately well-demarcated, nonencapsulated, moderately cellular, and highly infiltrative. Neoplastic cells are arranged in a lepidic growth pattern along pre-existing alveolar septa and also form disorganized acini and clusters. Alveoli are expanded by lakes of mucin with paucicellular neoplastic cells aligned along remnant alveolar septa. Neoplastic cells are round to polygonal with variably distinct cell borders and frequently exhibit large intracytoplasmic clear vacuoles which marginate the nucleus (signet ring cells).
The nuclei contain one to three variably prominent eccentric nucleoli. The remainder of the cytoplasm is granular and moderately to deeply basophilic. Anisocytosis and anisokaryosis are moderate, and mitoses are rarely observed. Neoplastic cells are frequently clustered around medium sized vessels and expand and disrupt the tunica media and adventitia. There is moderate infiltration of neoplastic foci and alveoli with large foamy alveolar macrophages containing mucin and fewer lymphocytes. Alveoli frequently contain small amounts of eosinophilic proteinaceous fluid (edema) and fibrin. Alveolar septa are expanded in multiple areas by neoplastic cells, collagen fibers, edema, and inflammatory cells including lymphocytes and macrophages.
Contributor’s Morphologic Diagnosis:
Lung: Metastatic duodenal mucinous adenocarcinoma.
Contributor’s Comment:
This marmoset was euthanized following chronic clinical decompensation that was unresponsive to medical management. The clinical differential diagnoses in this case included Marmoset Wasting Syndrome (MWS), infectious enteropathies, and enteric neoplasia due to the thin body condition, diarrhea, bony lesions, and progressive hypoalbuminemia. Proximal duodenal mucinous adenocarcinoma with both local invasion and distant metastasis was diagnosed in this case, and wasting was attributable to chronic neoplastic and inflammatory changes of the intestine which likely impaired absorption and resulted in malnourishment.
Reports of small intestinal adenocarcinomas are uncommon in the gastrointestinal tract of aged nonhuman primates despite those of the large intestine being among the most frequently observed.1,2,8,9 One limited case series and infrequent case reports have documented small intestinal adenocarcinomas in the marmoset.1,3
In marmosets, small intestinal adenocarcinomas typically begin as carcinomas in situ at the level of the jejunum or ileum before infiltrating the intestinal wall, and histology often demonstrates signet ring morphology and mucinous matrix.3,4 Local invasion and metastasis to mesenteric lymph nodes were reported in 6/10 cases of marmoset small intestinal adenocarcinoma, but distant metastases were not noted.3
The proximal duodenal localization in this case is atypical, as is the extensive metastatic disease. In this case, metastases were noted in the lungs, liver, and parapatellar adipose.6 Additionally, the primary tumor and all metastases demonstrated absent to attenuated expression of pancytokeratin, indicating active neoplastic epithelial-mesenchymal transition and suggesting a poorer prognosis.6 This case highlights the importance of considering infiltrative adenocarcinoma as a differential in captive marmosets exhibiting refractory weight loss and diarrhea.
Contributing Institution:
Johns Hopkins University
Department of Molecular and Comparative Pathobiology
Baltimore, MD 21230
https://mcp.bs.jhmi.edu/
JPC Diagnosis:
Lung: Mucinous adenocarcinoma, metastatic.
JPC Comment:
Adenocarcinomas arising in the small and large intestines of marmosets are rare and are thought to share a similar pathogenesis: dysregulation of the Wnt/ β-catenin signaling pathway.4 This pathway is an evolutionarily conserved signaling cascade that regulates myriad cellular processes, including cell proliferation, differentiation, and apoptosis.7 Under normal homeostatic conditions, β-catenin is subject to constitutive phosphorylation mediated by a complex of proteins, including the tumor suppressor gene product adenomatous poliposis coli (APC). The phosphorylation of β-catenin leads to its ubiquitination and subsequent proteosomal degradation, and prevents its accumulation in the cytosol.7
The activation of the canonical Wnt/ β-catenin pathway begins with the binding of the signaling ligand Wnt to Frizzled receptors at the plasma membrane. This binding recruits a variety of intracellular signaling proteins, including Disheveled and the APC complex, to the Frizzled receptor and inhibits the constitutive phosphorylation of β-catenin. Released from inhibition, β-catenin then accumulates in the cytosol, translocates to the nucleus, and activates Wnt-dependent gene expression.7 In human colorectal cancers, this signaling sequence is most commonly perturbed by loss-of-function mutations in APC, leading to loss of β-catenin phosphorylation and degradation and, consequently, to unchecked cellular proliferation.
β-catenin also exerts influence outside of the Wnt signaling pathway through its interactions with cadherins, transmembrane proteins that mediate cell adhesion by linking the actin cytoskeletons of neighboring cells. β-catenin binds to the cytosolic domains of cadherins and stabilizes these normal cell to cell anchoring interactions. An increase in cytosolic β-catenin upsets this balance and can result in loss of cohesion among cells, a necessary precondition for metastasis.4
Though similar oncogenic mechanisms are at work throughout the intestinal tract, adenocarcinomas are far more likely to arise in the large intestine than in the small intestine.
Factors currently thought to account for this disparity include: the faster transit time of digesta through the small intestine which decreases exposure to toxins when compared to the large intestine; the higher levels of IgA and mucosal-associated lymphoid tissue providing immune surveillance in the small intestine; and the increased distance between small intestinal crypt stem cells and the intestinal lumen which decreases exposure to luminal carcinogens.4
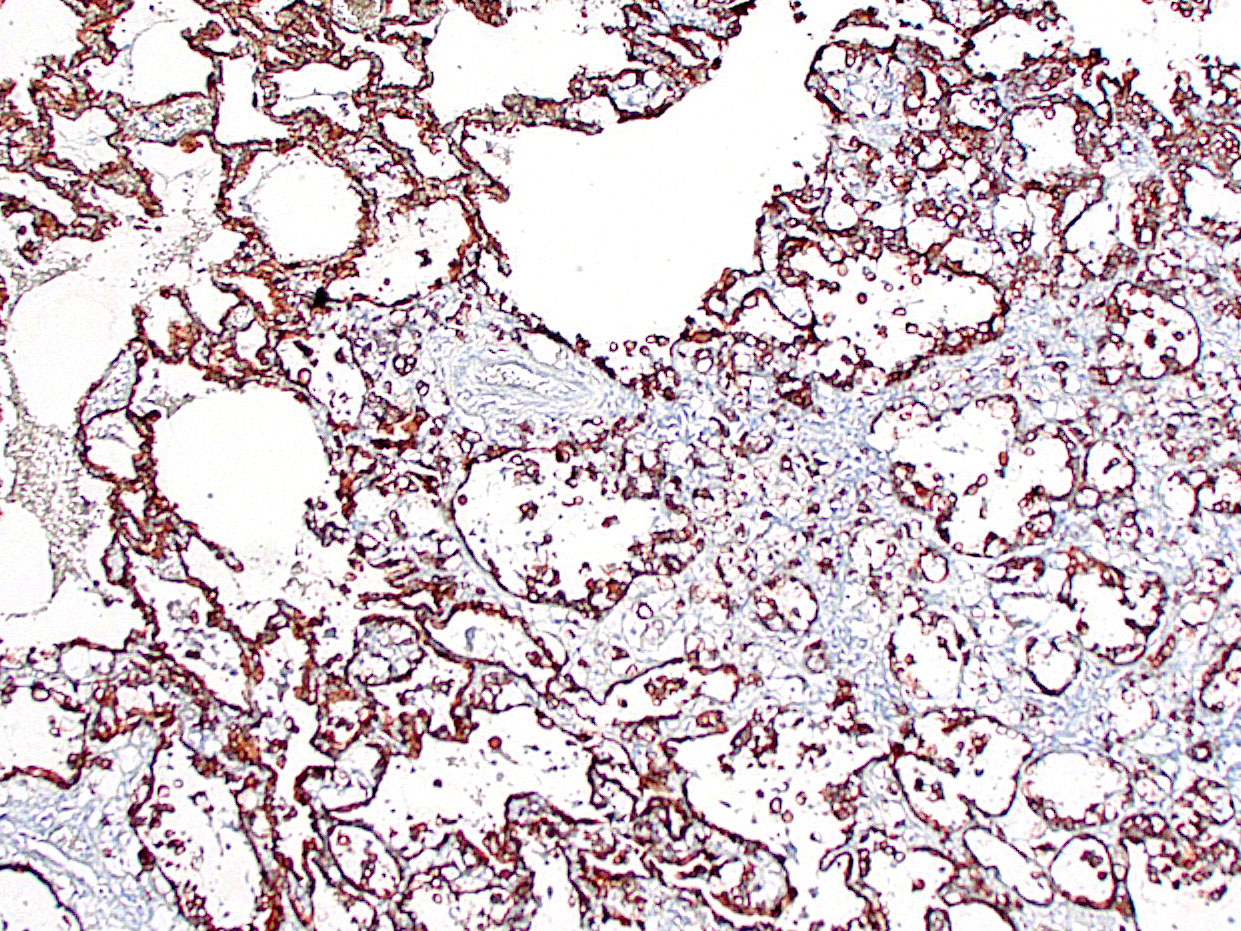
This case provoked a robust discussion among conference participants, several of whom felt they could not definitively rule out a primary pulmonary origin for this tumor. Immunohistochemical stains for TTF-1 and AE1/AE3 were examined during conference and largely served to confound. Though the contributor reported absent or attenuated pancytokeratin immunoreactivity, AE1/AE3 staining performed at the Joint Pathology Center revealed diffuse, strong cytoplasmic reactivity within the neoplastic cell population (Fig 3-6). The neoplastic cells were diffusely negative for TTF-1, a marker of primary pulmonary adenocarcinoma; however, there are case reports of TTF-1 negative primary pulmonary mucinous adenocarcinomas, including one recent case report in a marmoset.5 Ultimately, the morphology of the tumor and the multifocal distribution in the lungs convinced participants that the tumor represented metastatic disease. Participants also briefly discussed the signet ring cells found through this section. Though interesting, signet ring cell morphology can also be found in melanomas and squamous cell carcinomas, among other tumors, and has no known prognostic significance.
References:
- Brack M. Gastrointestinal tumors observed in nonhuman primates at the German primate center. J Med Primatol. 1998;27:319–324.
- Johnson LD, Ausman LM, Sehgal PK, King NW. A prospective study of the epidemiology of colitis and colon cancer in cotton-top tamarins (Saguinus oedipus). Gastroenterology. 1996;110(1):102–115.
- Miller AD, Kramer JA, Lin KC, Knight H, Martinot A, Mansfield KG. Small intestinal adenocarcinoma in common marmosets (Callithrix jacchus). Vet Pathol. 2010;47(5):969-976.
- Miller AD. Chapter 18 - Neoplastic Diseases. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, eds. The Common Marmoset in Captivity and Biomedical Research. Academic Press 2019:305-309.
- Mineshige T, Inoue T, Kawai K, et al. Spontaneous pulmonary adenocarcinoma in a common marmoset (Callithrix jacchus). J Med Primatol. 2021;50:335-338.
- Peterson C, Plunkard J, Johanson A, Izzi J, Gabrielson K. Immunohistochemical characterization of a duodenal adenocarcinoma with pulmonary, hepatic and parapatellar metastases in a common marmoset (Callithrix jacchus). J Comp Pathol. 2021;189:1–7.
- Silva-Garcia O, Valdez-Alarcón JJ, Baizabal-Aguirre VM. Wnt/β-catenin signaling as a molecular target by pathogenic bacteria. Immunol. 2019;10:1-14.
- Simmons HA, Mattison JA. The incidence of spontaneous neoplasia in two populations of captive rhesus macaques (Macaca mulatta). Antioxid Redox Signal. 2011;14(2):221–227.
- Valverde CR, Tarara RP, Griffey SM, Roberts JA. Spontaneous intestinal adenocarcinoma in geriatric macaques (Macaca sp.). Comp Med. 2000;50(5): 540-544.