Signalment:
Gross Description:
Morphologic Diagnosis:
Lab Results:
CBC: WBC 11.1 k/μl (â), neutrophils 8.87 k/μl (80.2 %), lymphocytes 1.48 k/μl (13.4 %), monocytes 0.623 k/μl (5.63 %), eosinophils 0.002 k/μl (0.014 %), basophils 0.086 k/μl (0.779 %), RBC 4.56 M/μl, hemoglobin 10.2 g/dl(â), HCT 30.7% (â), MCV 67.3 fl (â), MCH 22.4 pg (â), MCHC 33.3 g/dl, RDW 26.9 % (â), platelets 884.0 k/μl (ââ), MPV 12.2 fl; mild leukocytosis; mild anemia.
Bacteriology: Lungs: S. aureus +, S. intermedius +; kidney: E. coli ++; liver: E. coli +; spleen: negative; duodenum: Bacteroides spp. +; jejunum: Bacteroides spp. +, Streptococcus spp. (+); ileum: Streptococcus spp. (+); colon: E. coli +, Streptococcus spp. +, Bacteroides spp. ++.
Fluorescent antibody test (FAT): Direct immunofluorescence on cryosections of the small intestine was negative for coronavirus, but positive for rotavirus antigens.
Virology: By RT-PCR a 433 bp long fragment of genome segment 6 of group A rotavirus was amplified in RNA obtained from a jejunum sample.(6)
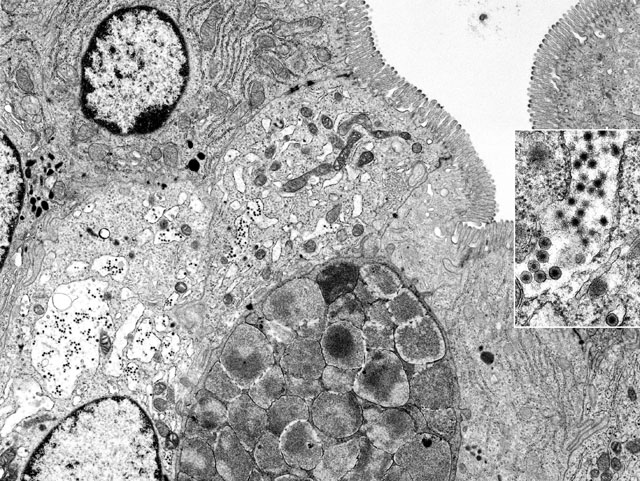
Electron Micrograph Description: Small intestine, mid-jejunum, fixed in glutaraldehyde, transmission electron micrograph, 11,800X: The image is composed of at least six intestinal absorptive epithelial cells which are characterized by numerous, even, long, plush microvilli along their luminal borders. The terminal web, especially of the two most lateral absorptive cells, shows immediately beneath the microvilli, few electron-dense filamentous structures extending into the cores of the microvilli. Within the cytoplasm there are numerous mitochondria, abundant rough endoplasmic reticulum (RER), occasional lysosomes and few spherical electron-dense vesicles. Three nuclei are present. The lateral cellular borders of the slightly interdigitating enterocytes show apical junctional complexes. Between two absorptive cells there is one goblet cell liberally endowed with characteristic electron-lucent mucous granules. In the center of the image there are two absorptive cells with more electron-lucent and brightened cytoplasm and a disorganized terminal web. Most strikingly is the severe dilation of the RER which has almost completely lost the ribosomes (ribosomal detachment). Within the dilated cisternae of RER there are numerous electron dense viral particles. The microvilli of these cells appear to be less dense and slightly out of register; mitochondria show lysis of cristae and a moderately condensed matrix.
Inset: Within the dilated cisternae of the RER numerous virus particles with envelope and mature virions after loss of the envelope, with a diameter of 60-80 nm, are present with ultrastructural features identical with those of rotaviruses (47,000X).
Condition:
Contributor Comment:
Rotaviruses are the causative agents of severe gastroenteritis in animals and humans and also have the potential to be transmitted between species. They are one of the most important infectious agents of severe diarrhea of infants and are associated with approximately 870,000 deaths per year in children under three years of age in developing countries.(2) Rotaviruses infect differentiated epithelial cells of the villi in the jejunum of a multitude of species including calves, piglets, foals, lambs, and avian species. The results are destruction of enterocytes at the apices of the villi of the small intestine, atrophy of the infected villi, restricted absorption, and increased secretion after neurogenic stimulation with a massive loss of liquid and electrolytes. The main clinical signs are diarrhea (white scours), weakness and anorexia. Young animals may die as a result of dehydration or secondary bacterial infection. (7)
Infections with rotaviruses are economically important, especially in younger animals one to eight weeks of age. In calves and pigs, lethal infections are seen during the first months of age. In contrast, infections of human newborns are usually relatively mild and asymptomatic. In humans, an increase in the incidence of infections and the severity of the clinical signs with fever and anorexia is seen not before several months of age. Most of the rotavirus infections with gastroenteritis in children are seen between the ages of six months and two years.(2)
Rotavirus is a nonenveloped virus; therefore, its stability and infectivity at temperatures of 18-20 °C is very high. Rotaviruses are also relatively resistant to treatment with formaldehyde, ether or detergents. Viral particles may remain infectious in the environment for several years.(2)
The genome of rotaviruses consists of double stranded, segmented RNA. The entire genome has 11 segments and in general a molecular weight of 11-12 x 106. Differences in the migration pattern of the single genome segments after elecrophoresis can be used for classification. Four groups (I-IV) of these electropherotypes can be differentiated. A distinct feature of rotavirus morphogenesis is that subviral particles, which assemble in the cytoplasmic viroplasms, bud through the membrane of the RER, and maturing particles are transiently enveloped. This is one of the most interesting aspects of rotavirus replication.(2,3)
Serologically, rotaviruses are divided into seven distinct groups (A-G). There is no correlation between the electropherotype and the serotype or group specificity of rotaviruses. Within the genus Rotavirus most of the characterized rotavirus isolates harbor a common group antigen. They are classified as group A. Isolates with similar replication characteristics, but which lack the group specific antigen are classified in additional groups (e.g. B, C, D, and E). Due to numerous additional characteristics of isolates belonging to one of the five groups, rotaviruses are subdivided into several subtypes. Rotavirus infections in cattle most often belong to group A. In humans infections are caused by viruses of group A or B. Rotavirus infections in pigs are often caused by viruses belonging to group B, C, or E. Infections in birds belong mainly to group D. Due to the large variety of rotavirus serotypes and subtypes present in the different species, animals and humans can be infected within a short time period with different serotypes.(4)
JPC Diagnosis:
Conference Comment:
In addition to three week scours or white scours in pigs, there are several other distinct syndromes in animals attributed to rotaviral infection, including infectious diarrhea of infant rats (IDIR), enzootic diarrhea of infant mice (EDIM), and diarrhea in young rabbits, calves, lambs, foals, puppies, kittens and poultry. Among these syndromes, rotaviral infection is generally characterized by several consistent themes, including a propensity to cause clinical disease in only young animals, a tendency to cause mild disease, and a predilection for infection of enterocytes at the villar tips.(1,5) That said, several intricacies regarding rotaviral infection in certain species warrant elaboration. In rats and mice respectively, IDIR and EDIM are both characterized by diarrhea in neonates less than two weeks old, with only subclinical infection in older animals. Infectious diarrhea of infant rats is caused by an atypical (i.e. non- Group A) rotavirus that is likely of human origin. In addition to intestinal villus attenuation and enterocyte necrosis that is typical of other rotaviral infections, IDIR is characterized by epithelial syncytia that are considered pathognomonic and may contain eosinophilic intracytoplasmic viral inclusions. In both wild and laboratory mice, EDIM is caused by a single, highly contagious strain of Group A rotavirus and results in transient diarrhea that may or may not be clinically significant.(5) In both lambs and rabbits, rotaviral infection is often seen in combination with bacterial co-pathogens (e.g. E. coli); lambs are unique in that viral infection of the colon may occur in this species, whereas in other species the small intestine is affected, with the specific site (i.e. duodenum, jejunum, or ileum) varying by species.(1,5)
Most conference participants considered coronaviral enteritis (i.e. transmissible gastroenteritis [TGE]) in the differential diagnosis for this case. The signalment and clinical signs of coronaviral and rotaviral infections are analogous, although the latter is generally less severe and less frequently characterized by vomiting. The gross and histopathologic findings are also comparable between the two entities, and both cause lesions whose severity is inversely proportional to age in piglets. Similarly, the microscopic lesions in the small intestine of calves with rotaviral enteritis are identical to those of calves with coronaviral enteritis; however, the former does not cause colonic lesions. Coronaviruses have a single-stranded RNA genome, are enveloped, and measure slightly larger than rotaviruses, ranging from 70-200 nm in diameter and averaging 100-130 nm in diameter. The characteristic corona of peplomers for which coronaviruses are named is best visualized ultrastructurally in negatively stained preparations.(1)
References:
2. Estes MK, Kapikian AZ: Rotaviruses. In: Fields Virology, eds. Knipe DM, Howley PM, 5th ed., pp. 1917-1974, Lippincott Williams & Wilkins, Philadelphia, PA, 2007
3. Granzow H, Schirrmeier H, Beyer J, Lange E: Morphologische Studien bei Virusinfektionen des Darmtraktes Virusreplikation und Zytopathologie in Zellkulturen und Enterozyten beim Ferkel, 1. Mitteilung: Ultrastruktur des nichtinfizierten Darmepithels und bei Rotavirusinfektionen. [Morphologic studies of virus infection of the intestinal tract--virus replication and cytopathology in cell cultures and enterocytes in piglets. 1. Ultrastructure of intestinal epithelium without viral infiltration and in rotavirus infection]. Arch Exper Vet Med 42:558-70, 1988
4. Matthijnssens J, Ciarlet M, Rahman M, Attoui H, B+�-�nyai K, Estes MK, Gentsch JR, Iturriza-G³mara M, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Patton JT, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Desselberger U, Van Ranst M: Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol 153:1621-1629, 2008
5. Percy DH, Barthold SW: Pathology of Laboratory Rodents and Rabbits, 3rd ed., pp. 42-43, 135-136, 261-262. Blackwell Publishing, Ames, IA, 2007
6. Schwarz BA, Bange R, Vahlenkamp TW, Johne R, M+�-+ller H: Detection and quantitation of group A rotaviruses by competitive and real-time reverse transcription-polymerase chain reaction. J Virol Methods 105:277-285, 2002
7. Yuan L, Stevenson GW, Saif LJ: Rotavirus and reovirus. In: Diseases of Swine, eds. Straw BE, Zimmermann JJ, DAllaire S, Taylor D, 9th ed., pp. 435-454, Blackwell, Ames, IA, 2006