WSC 2023-2024 Conference 7 Case 3
Signalment:
11-year-old, male neutered domestic short hair, feline (Felis catus)
History:
The patient is an outdoor cat with a completed vaccination protocol and no other animals live in the same household. The cat developed skin lesions due to dermatophytosis and was treated with itraconazol. There was partial remission of the skin lesions; however, approximately five months later, the skin lesions progressed despite therapy.
Gross Pathology:
Clinical examination of the skin showed severe diffuse exfoliation and crust formation. In addition, the skin presented with mild to moderate multifocal erythema and follicular casts. The ear canal was filled with dry and brown secretions.
Laboratory Results:
Cytological examination of the skin crusts revealed numerous neutrophils and bacterial cocci. Trichogram examination revealed the presence of follicular casts. A skin scraping was negative for ectoparasites. Wood lamp examination was negative for fungal microorganisms. Blood evaluation revealed mild thrombocytopenia and a mild increase in ALT.
Chest radiographs revealed a mass in the thymic region. Cytologic evaluation of an ultrasound guided fine needle aspirate of the mass was diagnosed as epithelial thymoma.
Microscopic Description:
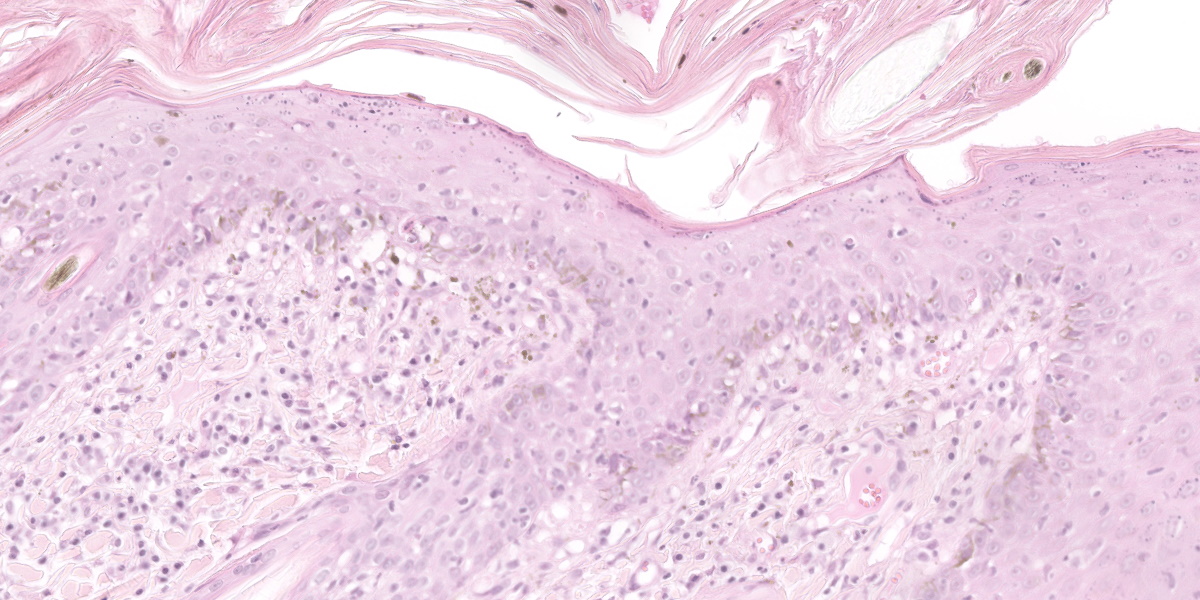
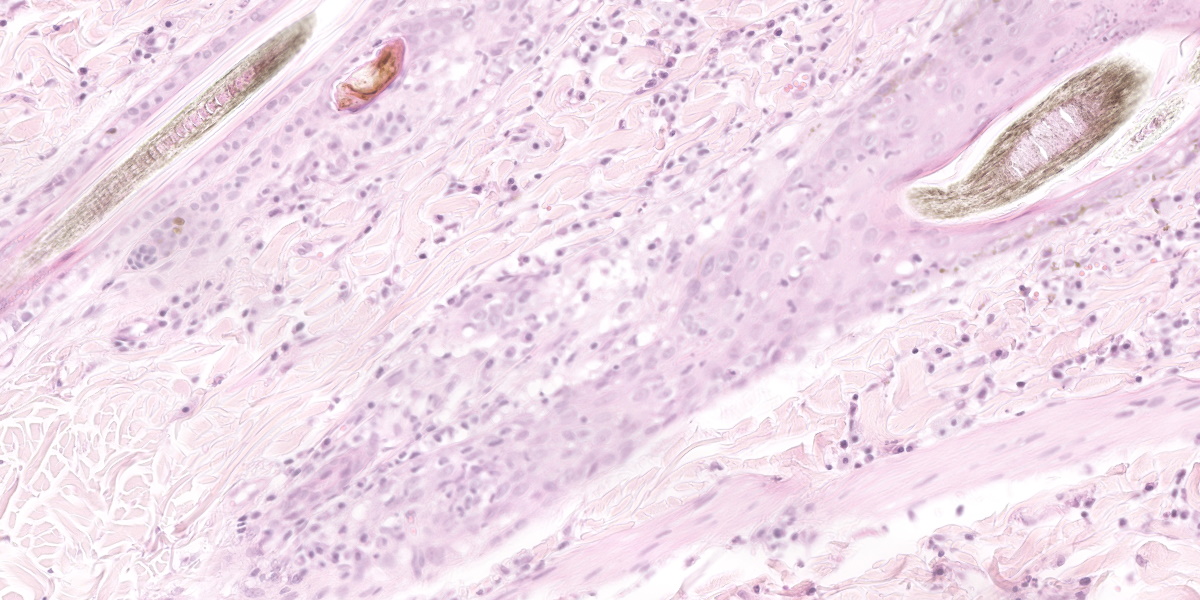
Histological examination of the skin sections revealed a marked, diffuse predominantly orthokeratotic and, to a lesser degree, parakeratotic hyperkeratosis of the epidermis. Multifocally, small aggregates of neutrophils as well as extravasated erythrocytes (haemorrhage) and occasional small bacterial colonies (coccoid bacteria, not seen in all slides) can be seen. The epidermis and hair follicle infundibula are moderately thickened (acanthosis). The following changes are seen in the epidermis and, to a lesser degree, in hair follicles: disruption and vacuolation (interpreted as hydropic degeneration) of the basal keratinocytes; an infiltrate of small lymphocytes within the stratum basale and rarely within upper layers (exocytosis); scattered isolated deeply eosinophilic and rounded cells (apoptotic cells) with loss of cohesion from other keratinocytes in all epidermal layers; increased numbers of intraepidermal melanocytes; and increased numbers of keratinocytes with intracytoplasmic melanin (hyperpigmentation). A moderate band-like mononuclear infiltrate composed mainly of lymphocytes, plasma cells, occasional melanin-laden macrophages (interpreted as melanin incontinence), and mast cells is seen in the superficial dermis and around hair follicles. This infiltrate obscures the dermo-epidermal junction (interpreted as interface dermatitis). Sebaceous glands are absent.
Contributor?s Morphologic Diagnosis:
Haired skin: Moderate to severe lymphocytic interface dermatitis with mild transepidermal apoptosis, moderate to severe parakeratotic hyperkeratosis, and severe atrophy of sebaceous glands.
Contributor?s Comment:
Thymoma associated exfoliative dermatitis is a rare paraneoplastic syndrome which has been reported in middle-aged to older cats affected by a thymoma.7,12 This condition has also been reported in rabbits and goats.1,5 In cats, the exfoliative dermatitis regresses after thymectomy.2,7,13 Other thymoma associated paraneoplastic syndromes include myasthenia gravis in cats and dogs, erythema multiforme in dogs, and granulocytopenia in cats.4,13,15,16
Affected cats present with alopecia, crusting, scaling, desquamation, and erythema usually starting from the head and progressing to the rest of the body.2,6,12 Keratinaceous debris may build up in interdigital spaces and claw folds. Pruritis is usually absent unless there secondary bacterial or yeast infection.7 Clinical signs in cats may include lethargy, anorexia, coughing, and dyspnoea.2,12 Similar are dermal lesions, namely alopecia and exfoliation, are reported in goats and rabbits.1,5
Typical histopathological lesions in cats include orthokeratotic and parakeratotic hyperkeratosis, extensive desquamation and transepidermal keratinocyte apoptosis, as well as apoptosis of keratinocytes in the follicular infundibula. In addition, there is hydropic degeneration of the basal keratinocytes and cell poor to cell rich CD3+ lymphocytic interface dermatitis. The infiltrative lymphocytes can lead to mural folliculitis with subsequent atrophy of sebaceous glands. Pigmentary incontinence and concurrent Malasezzia infections have also been reported.7,12 Similar histologic lesions have been described in goats and rabbits.1,5
In humans, thymomas have been shown to produce autoantigen responsive CD4+ T cells. This has been shown to occur in paraneoplastic myasthenia gravis, erythema multiforme, and graft versus host disease. It is therefore proposed that the same occurs in thymoma associated exfoliative dermatitis in cats due to the occurrence of auto reactive T lymphocytes targeting keratinocytes.12 Differential diagnoses of thymoma associated exfoliative dermatitis in cats according to histological features of the lesion include non-thymoma associated exfoliative dermatitis, systemic lupous erythematosus, erythema multiforme, and sebaceous adenitis.7
Nonthymoma associated exfoliative dermatitis is clinically and histologically indistinguishable from the thymoma associated exfoliative dermatitis.3,10 The diagnosis is based on the demonstration of the absence of thymic neoplasia and the lesions usually resolve after administration of cyclosporine.3,10,14
Erythema multiforme has similar histologic lesions to thymoma-associated exfoliative dermatitis. The lesions in thymoma associated exfoliative dermatitis present with milder transepidermal apoptosis in comparison to erythema multiforme.6,7,12 In addition, the clinical skin lesions differ and are mainly characterised by erythematous macules, papules or plaques over the dorsum and spreading peripherally.7,8
Lupus erythematosus is considered another differential due to histologic similarities. The difference is that in lupus erythematosus, there is usually a more prominent interface dermatitis and mild apoptosis restricted to the basal cells.7 Gross lesions in lupus erythematosus include erosions and ulcerations on the face, particularly on the nose.8
Feline sebaceous adenitis is considered a differential when the sebaceous glands are absent or reduced in number. However, the inflammation will be confined to the follicular isthmus and apoptosis is absent in sebaceous adenitis.7 A final diagnosis of thymoma-associated exfoliative dermatitis is achieved by evaluation of skin biopsies and demonstration of simultaneously occurring thymic neoplasia.1
Contributing Institution:
Institute of Veterinary Pathology
Vetsuisse-Faculty, University Zurich
Winterthurerstrasse 268
8057 Zurich, Switzerland
JPC Diagnosis:
Haired skin: Dermatitis and mural folliculitis, lymphocytic, cytotoxic interface, moderate, with epidermal hyperplasia, parakeratotic hyperkeratosis, and sebaceous gland loss.
JPC Comment:
Thymomas are rare in dogs and cats and arise from the epithelium of the thymus. Curiously, thymomas are classified as benign or malignant based on their ability to be resected at surgery, and not based on histopathologic criteria.13 Regardless of classification, metastasis is uncommon and recurrence after surgical resection is rare.13
Patients with thymomas may seek veterinary care due to respiratory symptoms from the primary tumor or for clinical signs referrable to any one of several reported paraneoplastic syndromes. As the contributor notes, the most common thymoma-associated paraneoplastic syndromes include exfoliative dermatitis, erythema multiforme, myasthenia gravis, granulocytopenia, and polymyositis. The association between thymoma and exfoliative dermatitis is poorly understood; however, immune-mediated injury is the current leading theory based on the characteristic presence of a T cell-rich cytotoxic interface dermatitis.12
In health, T cells are prevented from attacking self-antigens due to central and peripheral tolerance. Central tolerance begins during T cell development in the thymus, where rearrangement of T cell receptors generates a panoply of epitope receptors sufficient to recognize all possible antigens encountered during the life of the animal. In this generative process, T cell receptors are inadvertently created which have a high affinity for self-peptides. These T cells are screened in the thymic medulla and are either eliminated via apoptosis (?clonal deletion?) or differentiated into thymically-derived T regulatory cells (?clonal diversion?).11
Screening of self-reactive T lymphocytes is achieved through a remarkable process of so-called ?promiscuous gene expression? by a subset of thymic epithelial cells called medullary thymic epithelial cells (mTECs).11 These mTECs present self-antigens to developing T lymphocytes and expose maturing T lymphocytes to a nearly complete representation of the animal?s protein coding genome.11 T lymphocytes that pass this screening (i.e., have low binding affinity for self-peptides) are tolerant for most proteins in the animal?s body.11 A key player in this system is the transcriptional regulator Autoimmune Regulator (AIRE) protein, which is highly expressed in mTECs where it promotes promiscuous gene expression of a wide array of tissue-specific antigens for presentation to the developing T lymphocytes.
The processes of central tolerance work well, but not perfectly; thus, occasionally self-reactive T lymphocytes slip through the thymic cracks and are released into systemic circulation. In addition, though the mTECs present developing T cells with an impressive array of self-peptides, they do present them with all possible self-peptides. The processes of peripheral tolerance, most notably anergy and deletion of self-reacting lymphocytes, acts as a second line of defense against mature lymphocytes that encounter self-peptide for the first time outside the context of the thymus.11
Given that the thymus is the key site for establishing immune tolerance, it is not surprising that thymic epithelial cell tumors may generate syndromes caused by perturbations of this system. In humans, myasthenia gravis is the most studied thymoma-associated neoplastic syndrome. Patients that develop myasthenia gravis have cortical thymomas with active thymopoiesis (i.e., they retain the ability to export mature T lymphocytes).9 The neoplastic cells express lower levels of MHC Class II and AIRE when compared to normal thymic epithelium; thus, defective negative screening in the thymus, reduced levels of regulatory T cells, and export of autoreactive T lymphocytes are the key features associated with myasthenia gravis in human thymoma patients.9 As the contributor notes, it is thought that a similar mechanism underlies the apparent auto-immune keratinocyte destruction characteristic of thymoma-associated exfoliative dermatitis in a animals.
Conference discussion focused on distinguishing exfoliative dermatitis from erythema multiforme, as often the histologic lesions can be extremely similar or even identical. The clinical picture in these two conditions is different, however, with erythema multiforme typically causing small multifocal lesions while exfoliative dermatitis tends to be more widespread throughout the body.
Another differential considered, largely due to the loss of sebaceous glands, is sebaceous adenitis; however, involvement of the epidermis in the form of cytotoxic interface dermatitis in this case rules out a diagnosis of sebaceous adenitis, which is characterized by immune destruction of sebaceous units only. The loss of sebaceous units probably does contribute to the exfoliation in this condition, however, as the loss of sebum is a known cause of disordered desquamation. Conference participants discussed several other paraneoplastic syndromes, including paraneoplastic alopecia, nodular dermatofibrosis in German Shepherd dogs, paraneoplastic pemphigus, and superficial necrolytic dermatitis.
There was spirited discussion among conference participants surrounding the term ?diffuse? when used to describe distribution in dermatopathologic lesions. The moderator prefers to avoid the term as ?nodular to diffuse? dermatitis is one of the eight defined inflammatory reaction patterns in veterinary dermatopathology and its use in a broadersense may be confusing. In this case, the moderator felt that the terms ?dermatitis? and ?mural folliculitis? are sufficient to convey the distribution of the lesions examined in conference.
References:
- Byas AD, Applegate TJ, Stuart A, Byers S, Frank CB. Thymoma-associated exfoliative dermatitis in a goat: case report and brief literature review. J Vet DiagnInvest. 2019;31(6):905-908.
- Cavalcanti JV, Moura MP, Monteiro FO. Thymoma associated exfoliative dermatitis in a cat. J Feline Med Surg. 2014; 16(12):1020-1023.
- Combarros D, Moulin JP, Correge S, Delverdier M, Cadiergues MC. Clinical and histological recovery of non-thymoma-associated exfoliative dermatitis in a cat treated with cyclosporin A. JFMS Open Rep. 2020;6(1):205511692-0902 307.
- Fidel JL, Pargass IS, Dark MJ, Holmes SP. Granulocytopenia associated with thymoma in a domestic shorthaired cat. J Am Anim Hosp Assoc. 2008;44(4):210?217.
- Florizoone K. Thymoma-associated exfoliative dermatitis in a rabbit. Vet Dermatol. 2005;16(4), 281?284.
- Fournier Q, Bavcar S, Philbey AW, Smith S, Varjonen K. A previously undescribed cutaneous paraneoplastic syndrome in a cat with thymoma. Vet Dermatol. 2019;30(4):342-e98.
- Gross TL, Ihrke PJ,Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis. 2nd ed. Wiley-Blackwell;2008:68-79.
- Hnilica KA. Small Animal Dermatology: A color atlas and therapeutic guide. 3rd ed. Elsevier;2011.
- Lancaster E, Evoli A. Paraneoplastic disorders in thymoma patients. J Thorac Oncol. 2014;9(9 Suppl 2):S143-S147.
- Linek M, Rüfenacht S, Brachelente C, et al. Non-thymoma-associated exfoliative dermatitis in 18 cats. Vet Dermatol. 2015; 26(1):40-e13.
- Proekt I, Miller CN, Lionakis MS, Anderson MS. Insights into immune tolerance from AIRE deficiency. Curr Opin Immunol. 2017;49:71-78.
- Rottenberg S, von Tscharner C, Roosje PJ. Thymoma-associated exfoliative dermatitis in cats. Vet Pathol. 2004;41(4): 429-433.
- Singh A, Boston SE, Poma R. Thymoma-associated exfoliative dermatitis with post-thymectomy myasthenia gravis in a cat. Can Vet J. 2010;51(7): 757-760.
- Szczepanik M, Wilko?ek P, ?miech A, Kalisz G. Non-thymoma-associated exfoliative dermatitis in a European shorthair cat: A case report. Vet Med Sci. 2021; 7(6):2108-2112.
- Tepper LC, Spiegel IB, Davis GJ. Diagnosis of erythema multiforme associated with thymoma in a dog and treated with thymectomy. J Am Anim Hosp Assoc. 2011;47(2):e19-e25.
- Wood SL, Rosenstein DS, Bebchuk T. Myasthenia gravis and thymoma in a dog. Vet Rec. 2001;148(18):573-574.