Signalment:
Gross Description:
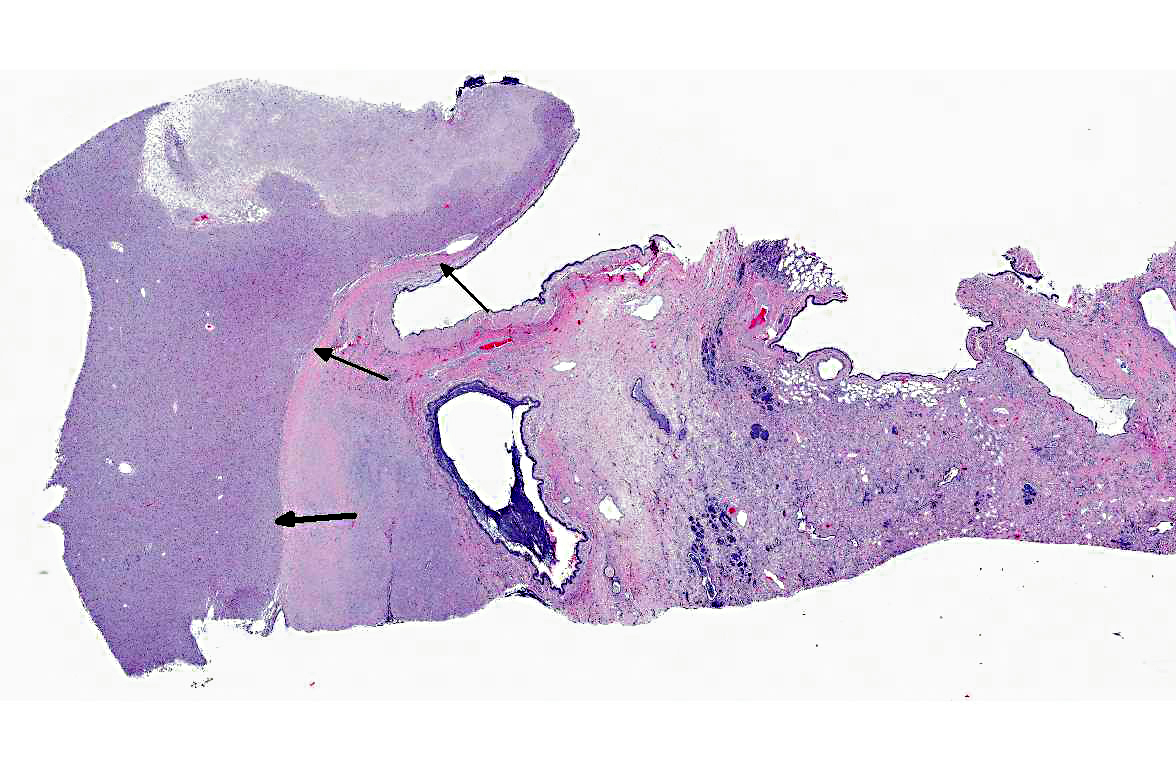
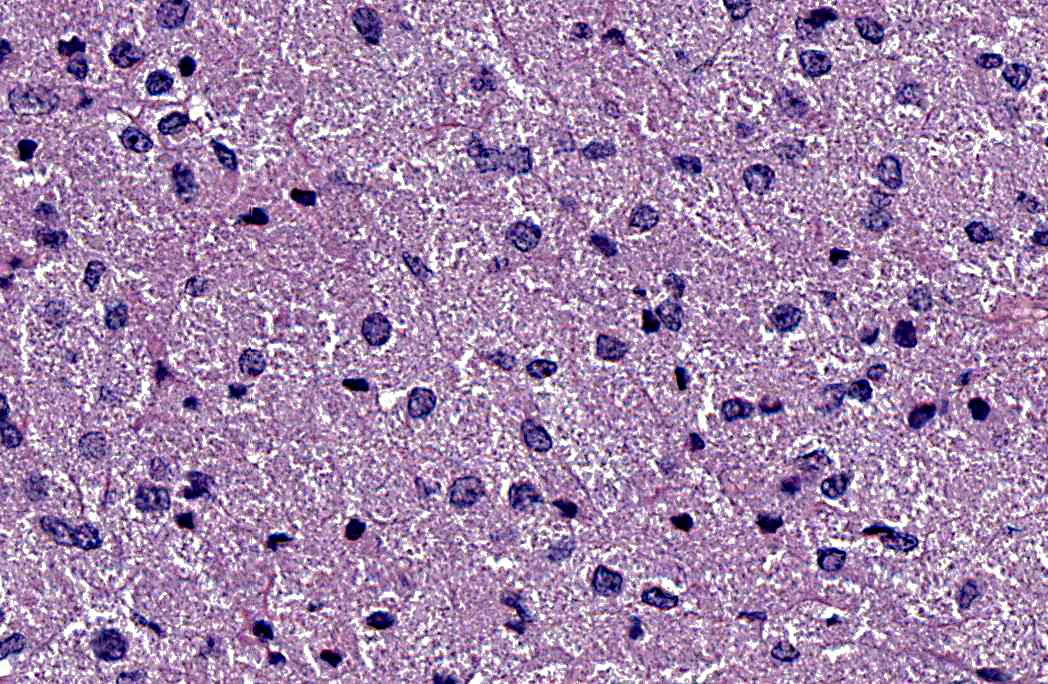
Histopathologic Description:
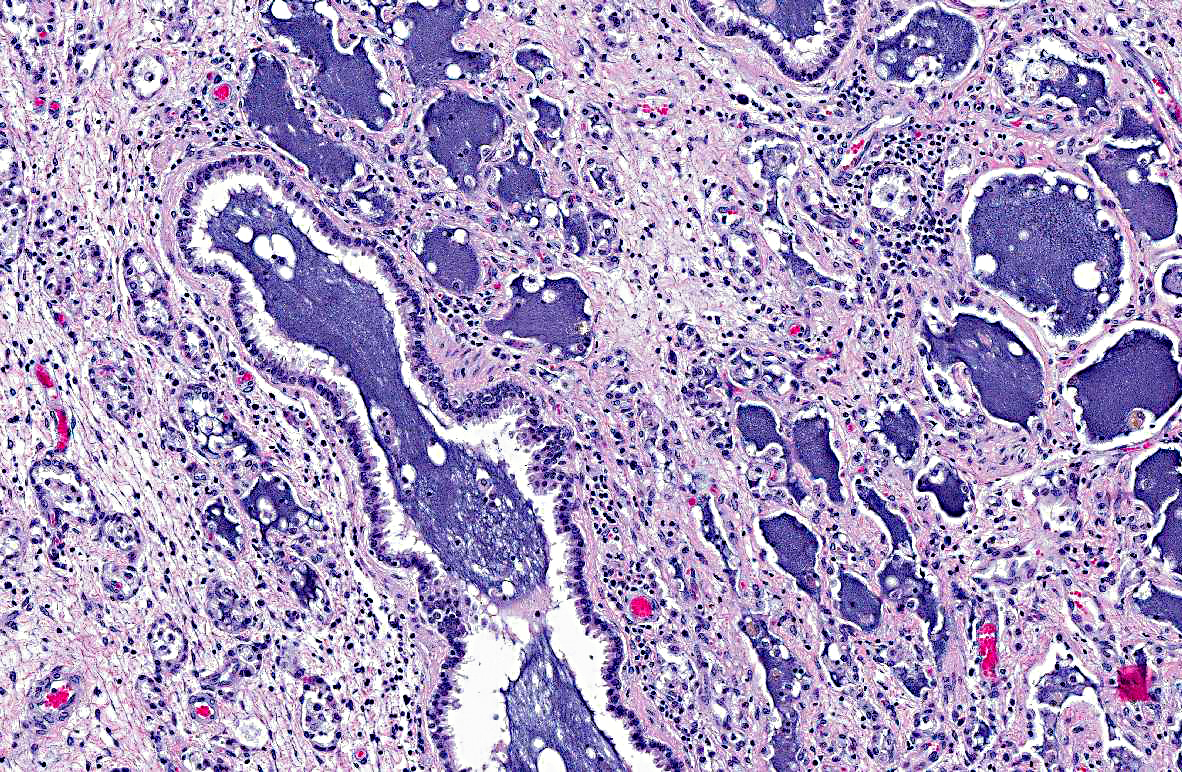
In the adjacent parenchyma, the interstitial septa are expanded (up to 10 times normal) by abundant fibrous connective tissue containing plump fibroblasts mixed with a moderate number of neutrophils, lymphocytes, plasma cells, and macrophages containing intracytoplasmic golden- yellow pigment (hemosiderophages). The bronchoalveolar spaces are filled with a moderate number of degenerate and intact neutrophils, lymphocytes, plasma cells, foamy macrophages, hemosiderophages, necrotic debris, and in few sections they contain granular to fibrillar amphophilic material (mucin). Epithelium lining the bronchioles and alveolar ducts is infiltrated by few neutrophils (exocytosis), and is degenerate (intracytoplasmic vacuolation). Most of the alveolar spaces are compressed by interstitial fibrosis and those remaining are lined by a single layer of low cuboidal epithelium (type II pneumocyte hyperplasia).
Morphologic Diagnosis:
1. Left lung: Granular cell tumor.
2. Left lung: Marked, diffuse, chronic-active, neutrophilic and lymphohistiocytic bronchointerstitial pneumonia with fibrosis.
Condition:
Contributor Comment:
Although previous reports in humans and in horses strongly support a Schwann cell or neural origin, the histogenesis of GCTs is still controversial.(2,4) While the light and electron microscopic appearance of GCTs in most species is similar, histochemical and immunohistochemical staining varies between the tumor site(s) and species, suggesting multiple cell origins.(2,4) GCTs are vimentin positive and inconsistently positive for S-100, neuron-specific enolase (NSE) and cytokeratin; however, they consistently stain PAS-positive and are diastase resistant. GCTs should be differentiated from other tumors with granular cytoplasm such as oncocytoma (PAS- positive, but sensitive to diastase digestion), rhabdomyoma (desmin- and myoglobin- immunopositive), chemodectoma/carcinoids (packets and nests of cells immunopositive for neuroendocrine markers) and large granular lymphocyte tumors.
In the present case, there was significant involvement of the left lung. Chronic-active pneumonia and fibrosis are secondary to obstruction of the major airways by the neoplasm and the consequential blockage/reduction of normal mucociliary clearance (a critical innate immune response) of pathogens.
JPC Diagnosis:
Conference Comment:
In a study of four horses with pulmonary GCTs, all tumors showed uniformly strong positive labeling with antibodies to vimentin, S100 protein, and glial fibrillary acidic protein (GFAP).3 Vimentin expression is common in cells of mesenchymal origin, and S100 is commonly found in cells of neural crest origin. GFAP is expressed by non-myelin forming Schwann cells in mice and humans. Although myelin-forming Schwann cells express both S100 protein and vimentin, this expression is not specific, as S100 and vimentin antibodies also react with some tumors of non-neural origin. Virtually all tumor cells in all four horses in this study showed positive immunoreactivity to myelin basic protein (MBP) and protein gene product 9∙5 (PGP9∙5). MBP antibodies react with components of Schwann cell-derived myelin. PGP9∙5 is a major component of neuronal cytoplasm; it is a reliable marker for neurons, and is used in the diagnosis of intraoral granular cell tumors in humans. A few tumor cells from all horses in this study also showed positive immunoreactivity with Leu7. Leu7 reacts with myelin-associated glycoprotein in human neuroectodermal tissue, and some human GCTs have been labeled with antibodies against specific neural markers such as MBP and Leu7. All four tumors lacked expression of neurofilament protein (NF), cytokeratin (CK), chromogranin, α1 antichymotrypsin (AACT), myoglobin, desmin, α-actin and α-SMA, ruling out neuroendocrine or myogenic cell origin. This study, along with others, supports the hypothesis that equine pulmonary GCTs are composed of myelinating and nonmyelinating Schwann cells.(3)
Conference participants uniformly agreed with the contributors conclusion that the chronic pulmonary pathology in the adjacent lung tissue is secondary to the neoplasm.
References:
2. Kelley LC, Hill JE, Hafner S, Wortham KJ. Spontaneous equine pulmonary granular cell tumors: morphologic, histochemical, and immunohistochemical characterization. Vet Pathol. 1995:32:101-106.
3. Lopez A. Respiratory system, mediastinum, and pleura. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St Louis MO: Elsevier Mosby; 2012:533.
4. Mittal KR, True LD. Origin of granules in granular cell tumor. Arch Pathol Lab Med. 1986;112:302-303.
5. Patnaik AK. Histologic and immunohistochemical studies of granular cell tumors in seven dogs, three cats, one horse, and one bird. Vet Pathol. 1993;30:176-185.
6. Troncoso P, Ordonez NG, Raymond AK, Mackay B. Malignant granular cell tumor: immunocytochemical and ultrastructural observations. Ultrastruct Pathol. 1988;12:137-144.
7. Van der Gaag I, Walvoort HC. Granular cell myoblastoma in the tongue of a dog: a case report. Vet Quart. 1983;5:89-93.
8. Wright JA, Goonetilleke URP, Waghe M, Stewart M, Carlile A. Comparison of a human granular cell tumour (myoblastoma) with granular cell tumours (meningiomas) of the rat meninges - an immunohistological and ultrastructural study. J Comp Pathol. 1990;103:191-198.