Signalment:
9-year-old, male, neutered, Golden Retriever mix dog (
Canis familiars).One year prior to presentation, the dog had developed epistaxis and a right nasal mass that was diagnosed as nasal adenocarcinoma following biopsy and histopathologic examination. The epistaxis responded well to treatment with tramadol, firocoxib, and Chinese herbs. In March of 2012, the dog presented for a 3-day history of progressive anorexia, diarrhea, vomiting, and lethargy. The dog was salmon fishing 10 days prior to admission and was found with a fish carcass. The owners elected empirical therapy and additional medications prescribed were meropitant and metronidazole. The dog re-presented two days later due to lack of improvement and development of dark colored diarrhea. On physical exam he was dull and panting, with a temperature of 99.8 degrees Celsius and a heart rate of 150 bpm. The right mandibular lymph node was moderately enlarged and there was dark brown fecal staining on fur around perineum. Due to the lack of response to the medications and continuing anorexia, the owners elected euthanasia and necropsy.
Gross Description:
Mucous membranes and abdominal fat were pale with a yellow tinge. There was an irregular, thin, undulating mass in the right ethmoid concha measuring 2 x 3 x 2 cm. On impression smear of the mass there were clusters of epithelial cells with anisokaryosis and prominent nucleoli and also macrophages with Neorickettsial organisms found intracytoplasmically. There was lymphadenopathy characterized by an enlarged right mandibular lymph node (2 x 1 x 2 cm), enlarged tracheobronchial lymph nodes (0.5 x .05 x 0.5 cm to 1 x 2 x 1 cm) with multifocal hemorrhages, and enlarged mesenteric lymph nodes (1 x 1 x 1 cm to 2.5 x 1 x 1.5 cm) also with multifocal hemorrhages.
On impression smears of the mesenteric and tracheobronchial lymph nodes there were large numbers of organisms consistent with
Neorickettsia within macrophages. The spleen was moderately enlarged with a vaguely cobblestone surface and was meaty on cut section.
Histopathologic Description:
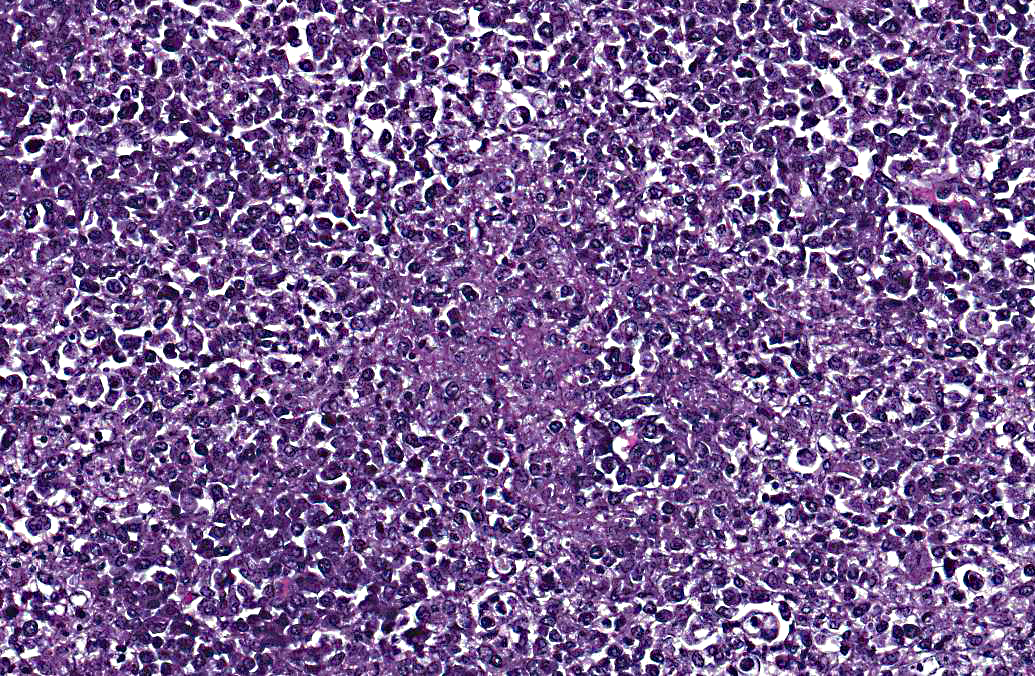
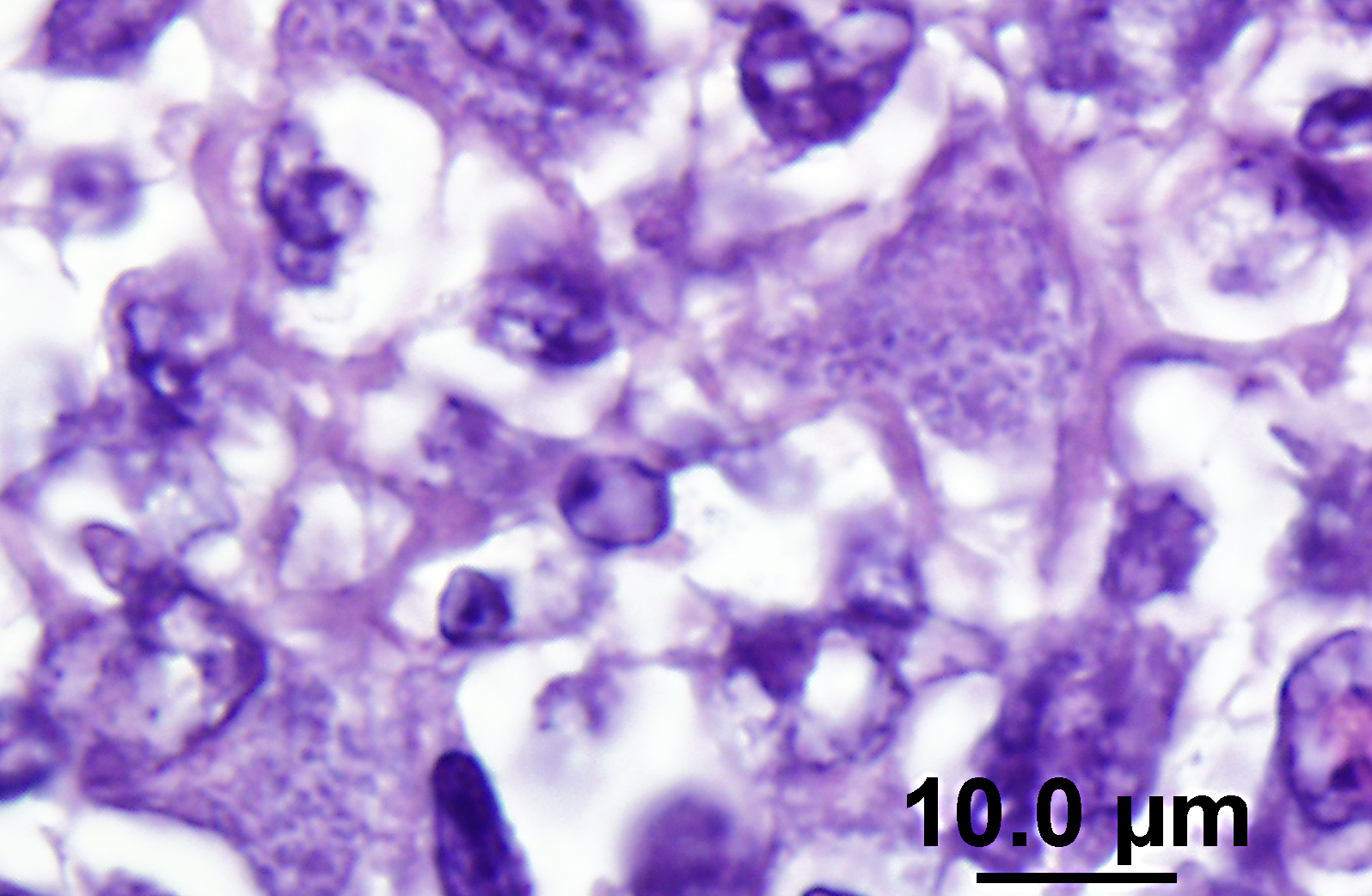
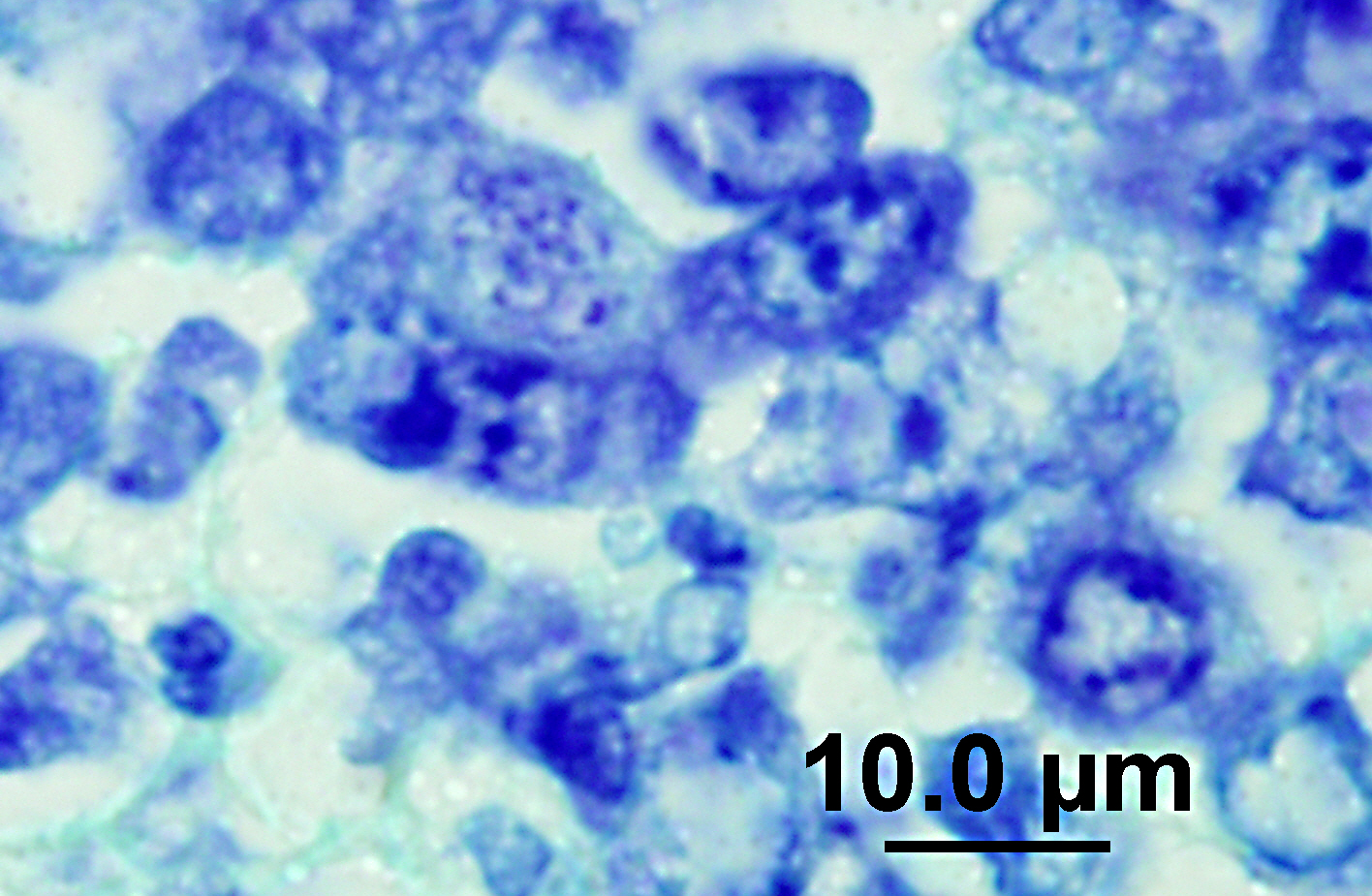
The lymph node is hyperplastic and hemorrhagic. The medulla is edematous often with large foamy macrophages filled with red blood cells (erythrophagocytosis). Macrophages are also often characterized by large, swollen, pale basophilic cytoplasm with numerous fine blue organisms. There are multifocal areas of necrosis characterized by leukocytes with pyknotic and karyorrhectic nuclei, cellular debris, occasional neutrophils and flocculent amphophilic debris. There are multifocal areas of hemorrhage. The cortex is packed with large histiocytes and fewer lymphocytes and plasma cells. Sinuses often contain macrophages displaying erythrophagocytosis. Subcapsular sinuses are congested often with erythrophagocytic macrophages and deposition of fibrillar to amorphous eosinophilic material deposits consistent with fibrin.
Morphologic Diagnosis:
Lymph node: granulomatous lymphadenitis with hemorrhage and intralesional rickettsial organisms (salmon poisoning-
Neorickettsia helminthoeca).
Lab Results:
Mildly increased ALP 298 U/L (RI: 10-84); CBC: Normal; U/A: Unremarkable; PCR: Lymph node and feces, positive for Neorickettsial spp real time PCR assay (still in validation process).
Condition:
Salmon poisoning
Contributor Comment:
Salmon poisoning disease (SPD) was first described in 1814 and occurs in northwest coastal regions including California, Oregon, Washington, and southern British Columbia.(10) Recently, SPD has been reported in Brazilian dogs confirmed with IHC and PCR for
N. helminthoeca.(5,6) The disease is caused by
Neorickettsia helminthoeca and the definitive host includes foxes, coyotes, and dogs; captive bears have also been reported to be highly susceptible to clinical disease after ingestion of trematode infected fish.(2,3,7)
N. helminthoeca require three hosts for life cycle completion.(6) The life cycle includes a trematode vector,
Oxytrema silicula, considered important in the geographic distribution of the disease, and a fish.(6) Miracidia of
Nanophyetus salmincola penetrate the fresh water snail,
Oyxtrema silicula, the cercariae then infect the second intermediate host by skin penetration or ingestion of infected snails or free cercariae.(1,4) The second intermediate host is most often salmon or trout.(1) The cercariae develop into metacercariae and become encysted in tissue of the salmon including eyes, kidneys, liver, intestine, fins and musculature.(1) Salmon in enzootic area are often heavily infected with the metacercariae.(1) Infection in dogs occur when uncooked fish is ingested and the metacercariae mature into flukes in the intestine.(1) The fluke does not cause the clinical signs but eggs appear in the feces of dogs five to ten days after infection and are a method of diagnosis.(1) The neorickettsial organisms, present in all stages of the fluke life cycle, spread via macrophages to visceral tissues of the dog causing disease.(1,8) The pathogenesis is still unclear in some ways, but the fluke attaches deep in the intestinal mucosa causing an inflammatory response and release of
N. helminthoeca by an unknown mechanism.(6) The
N. helminthoeca are taken up by intestinal macrophages and are disseminated through the lymph systems to lymph nodes where they proliferate with subsequent hypertrophy of the lymph nodes due to influx of macrophages and edema.(6) Enteritis is caused by the inflammation of the Peyers patches and intestinal lymphoid tissue.(6) The organism is found in circulation of infected dogs 8-12 days after infection.(3) Mortality rate is about 90% in untreated cases and death occurs at least 18 days after ingestion of metacercariae.(1,6) Dogs are immune to reinfection but only with the same strain. Subsequent infection of recovered dogs has been reported with alternate strains resulting in disease.(8,10) A similar but sometimes less severe disease has been described in the Elokomin River of Washington called Elokomin fluke fever.(6) This disease has a wider host range and the causative agent is thought to be another strain of
N. helminothoeca.(6,10)
Pathologic findings include enlarged mesenteric lymph nodes. Other nodes are usually less affected. Peyers patches are hypertrophic and the entire intestinal tract may be hemorrhagic.(6) Histopathologic findings are usually in the intestine and mesenteric lymph nodes with lymphoid tissue depletion and proliferation of lympho-histiocytic cells with neorickettsial bodies.(6) Giemsa or Machiavellos stains demonstrate organisms within macrophages.(6) Non-suppurative leptomeningitis and centrilobular fatty degeneration in the liver have also been described.(6) In this case there was a non-suppurative leptomeningitis and multifocal areas of hepatocellular vacuolation suggestive of lipid in the liver. The lymph nodes examined, including tracheobronchial and mesenteric, had obscured architecture with high cellularity and multifocal areas of necrosis with infiltration by large foamy macrophages containing neorickettsial organisms. There were similar findings in the spleen.
Diagnosis is based on a combination of fecal sedimentation and flotation, abdominal ultrasound, lymph node aspirate and PCR.(10) Identification of operculated trematode eggs via direct smear or washing-sedimentation in feces of infected dogs 5-8 days post infection or identification of the adult fluke within the intestine are methods of diagnosis.(6) Trematode infection may also occur in dogs recovered from salmon poisoning disease and during recovery.(6) Microscopic identification of neorickettsial bodies within macrophages may be performed on lymph node aspirates.(6) Definitive diagnosis includes isolation and culture of
N. helminthoeca, serology, and PCR.(6) In this case, PCR was performed on lymph node impression smears, serum, and feces and was positive in both lymph node aspirate and fecal sample but the serum was negative. Giemsa and Rickettsia-Pierce VanderKamp staining identified organisms in the macrophages in several tissues including the adenocarcinoma, multiple lymph nodes, spleen, intestine, and liver. In endemic areas this disease should be considered in dogs with clinical gastrointestinal signs such as anorexia, vomiting, and diarrhea as recognition of the disease and prompt treatment with a tetracycline type antibiotic is important for patient survival. Although it was known that the dog had been near salmon prior to development of clinical signs the owners were certain that the dog did not ingest any salmon. This case illustrates the importance of treatment with a tetracycline family antibiotic in dogs developing clinical signs of gastroenteritis when salmon exposure is known or suspected.
JPC Diagnosis:
Lymph node, mesenteric: Lymphadenitis, necrotizing, diffuse, moderate to severe with hemorrhage and intrahistiocytic coccobacilli.
Conference Comment:
The contributor provides a thorough and current review of salmon poisoning disease in dogs. Conference participants discussed the pathogenesis and temporal qualities of the histopathologic lesions in the submitted lymph node. The multifocal preponderance of plasma cells and the expanded paracortex are evidence of a reactive lymph node; however, the most significant feature is the marked necrosis, characterized by the loss of normal follicular architecture and replacement by necrotic debris admixed with fibrin, hemorrhage, numerous macrophages and fewer neutrophils, as well as multifocal, widespread lymphocytolysis. Participants hypothesized that this sample represents a necrotizing lymphadenitis due to
N. helminthoeca infection in a lymph node with pre-existing reactive hyperplasia. Additionally, the large amount of subcapsular and sinus hemorrhage was speculated to be due to draining from hemorrhagic enteritis, a feature commonly seen in this disease.
The genus
Neorickettsia, along with three other genera (
Anaplasma, Ehrlichia, and
Aegyptianella) comprise the family
Anaplasmatacea, within the order
Rickettsiales. Anaplasmatacea organisms are minute, non-motile Gram-negative obligate intracellular bacteria that lack cell walls and infect cells of hemopoietic origin. Generally, they cause arthropod-borne systemic disease in animals as well as humans.
Anaplasmatacea species of importance in veterinary medicine are summarized in the table below:(9)
| Pathogen | Host | Vector | Target cells | Disease |
| Aegyptianella pullorum | Poultry | Ticks | Erythrocytes | Aegyptianellosis |
| Anaplasma bovis | Cattle | Ticks | Erythrocytes | Aegyptianellosis |
| Aegyptianella pullorum | Poultry | Ticks | Monocytes, macrophages | Bovine anaplasmosis |
| Anaplasma marginale | Ruminants | Ticks | Erythrocytes | Anaplasmosis |
| Anaplasma ovis | Sheep, goats | Ticks | Erythrocytes | Anaplasmosis |
Anaplasma phagocytophilum
*Formerly Ehrlichia equi and Ehrlichia phagocytophila | Ruminants, horses, humans | Ticks | Granulocytes | Tick-borne fever, equine and human granulocytic ehrlichiosis |
| Anaplasma platys | Dogs | Ticks? | Platelets | Canine cyclic thrombocytopenia |
| Ehrlichia canis | Dogs | Ticks | Monocytes, macrophages | Canine monocytic ehrlichiosis |
| Ehrlichia ewingii | Dogs | Ticks | Granulocytes | Canine granulocytic ehrlichiosis |
| Ehrlicia (formerly Cowdria) ruminantium | Ruminants | Ticks | Granulocytes | Heartwater |
| Ehrlichia ondiri | Cattle | Ticks? | Granulocytes, monocytes | Bovine petechial fever |
| Ehrlichia ovina | Sheep | Ticks | Monocytes, macrophages | Ovine ehrlichiosis |
| Neorickettsia elokominica | Dogs, bears, raccoons | Flukes | Monocytes, macrophages, lymphoid cells | Elokomin fluke fever |
| Neorickettsia helminthoeca | Dogs, bears | Flukes | Monocytes, macrophages, lymphoid cells | Salmon poisoning disease |
| Nerickettsia risticii | Horses | Flukes | Monocytes, intestinal epithelial cells, mast cells | Potomac horse fever |
Table adapted from Veterinary Microbiology and Microbial Disease, 2nd ed, 2011.
References:
1. Booth AJ, Stogdale L, Grigor JA. Salmon poisoning disease in dogs on Southern Vancouver Island.
Can Vet J. 1984;25:2-6.
2. Foreyt WJ, Thorson S. Experimental salmon poisoning disease in juvenile coyotes (
Canis Latrans).
Journal of Wildlife Diseases. 1982;18(2):159-162.
3. Gai JJ, Marks SL. Salmon poisoning disease in two Malayan sun bears.
Journal of the American Veterinary Medical Association. 2008;232(4):586-588.
4. Gebhardt GA, Millemann RE, Knapp SE, Nyber PA. Salmon poisoning disease. II. Second intermediate host susceptibility studies.
The Journal of Parasitology. 1966;52(1):54-59.
5. Headly SA, Kano RS, Scorpio, DG, Tamekuni K, Barat NC, Bracarense AP, et al.
Neorickettsia helminthoeca in Brazilian dogs: a cytopathological, histopathological and immunohistochemical study.
Clinical Microbiology and Infection. 2009;15:21-23.
6. Headley SA, Scorpio DG, Vidotto O, Dumler JS.
Neorickettsia helminthoeca and Salmon Poisoning disease: A review.
The Veterinary Journal. 2011;187:165-173.
7. Johns JL. Salmon poisoning disease in dogs: A satisfying diagnosis.
The Veterinary Journal. 2011;187:149-150.
8. Nyberg PA, Knapp SE, Millemann RE. Salmon poisoning disease. IV. Transmission of the disease to dogs by
Nanophyetus salminocola eggs.
The Journal of Parasitology. 1967;53(4):694-699.
9. Quinn PJ, Markey BK, Leonard FC, FitzPatrick ES, Fanning S, Hartigan PJ. Rickettsiales and
Coxiella burnetii. In:
Veterinary Microbiology and Microbial Disease. 2nd ed. Ames, Iowa: Blackwell Science Ltd; 2011: Kindle edition.
10. Sykes JE, Marks SL, Mapes S, Schultz RM, Pollard RE, Tokarz D, et al. JE. Salmon poisoning disease in dogs: 29 cases.
J Vet Intern Med. 2010;24:504-513.