WSC Conference 4, Case 3
Signalment:
4-year-old Holstein cow, bovine (Bos taurus)
History:
The cow developed progressive weight loss, recumbency, weakness, depression, and mild hyperthermia. Despite treatment with an antibiotic (ceftiofur), a non-steroidal anti-inflammatory drug (flunixin), a corticosteroid (dexamethasone), and a parenteral solution containing vitamins, glucose and amino acids, her clinical condition deteriorated over a few weeks, necessitating euthanasia due to poor prognosis. The cow was pregnant at approximately 5 months of gestation.
Gross Pathology:
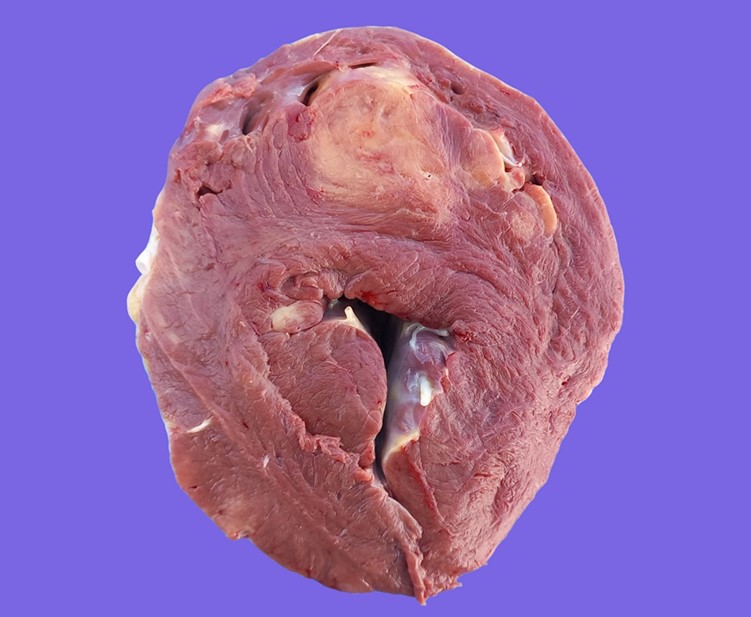
Multifocally infiltrating/expanding the myocardium and extending into the adjacent endocardium and epicardium in the atria, interventricular septum and the left ventricular free wall, there were multiple, yellowish/tan, irregular mass-like foci of approximately 1 to 8 cm, with poorly defined borders. In the myocardium, some of the larger foci exhibited a central 1–2 cm area of necrosis, demarcated by an incomplete thin (1–2 mm) red rim/halo (hemorrhage). The mesenteric lymph nodes were markedly enlarged, measuring up to 15 x 8 cm.
Laboratory Results:
In 2019, the cow had tested positive for serum antibodies against bovine leukemia virus (BLV) by ELISA and for BLV by qPCR on a sample of whole blood (6.45 x 104 BLV genome copies/µL), and a complete blood count revealed leukocytosis (white blood cells: 14.7 x 109/L; reference range: 5.8–12.6 x 109/L) with lymphocytosis (10.07 x 109/L; reference range: 1.7–5.6 x 109/L). A sample of heart obtained during the autopsy also tested positive for BLV by qPCR (1 x 106 genome copies/mg).
Microscopic Description:
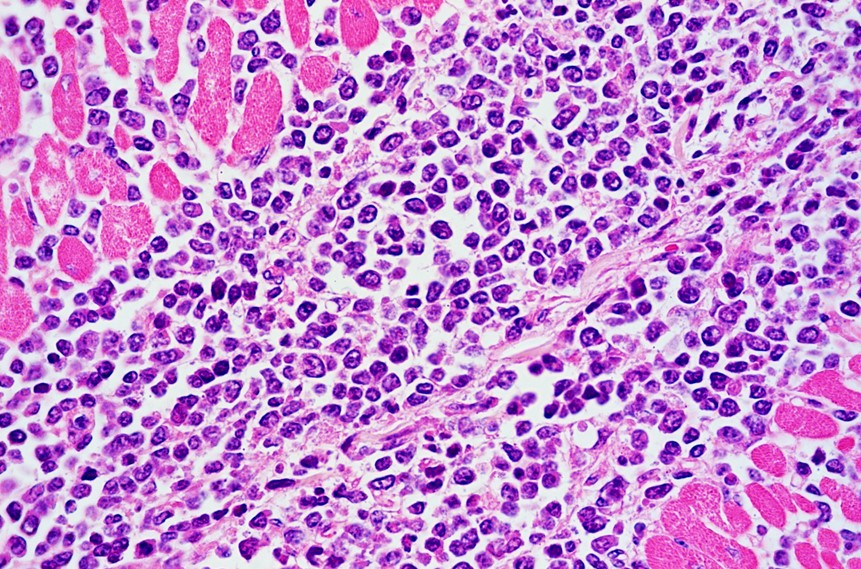
Two sections of heart are examined. Extensively infiltrating the myocardial interstitium including the endomysium, separating myocardial fibers, and multifocally infiltrating the endocardium and the conduction system, is an unencapsulated, densely cellular neoplasm composed of round cells arranged in sheets. Neoplastic cells are round to oval, with distinct borders, scarce cytoplasm, and high nuclear-to-cytoplasmic ratio. Nuclei are large, round, oval or reniform (moderate anisokaryosis), with coarse or clumped chromatin and 1 to 3 magenta nucleoli. The mitotic count is up to 8 per 400x field. Additionally, in one of the sections there is a fairly well-demarcated central area of necrosis that involves both the cardiomyocytes and the entrapped infiltrating neoplastic cells. In this area cardiomyocytes have hypereosinophilic and coagulated sarcoplasm, with loss of transverse striations (necrosis), neoplastic cells exhibit karyolysis, and there are occasional foci of erythrocyte extravasation (hemorrhages). Occasional intact, thick-walled, oval, basophilic, protozoal cysts morphologically resembling Sarcocystis spp. cysts are present within the sarcoplasm of cardiomyocytes.
Contributor’s Morphologic Diagnosis:
Heart: Lymphoma, holstein, bovine.
Contributor’s Comment:
The diagnosis of Enzootic Bovine Leukosis (EBL) in this cow was based on gross, microscopic, molecular (qPCR), and serological (ELISA) findings. The seroprevalence of BLV was high in the herd.
EBL is caused by BLV, genus Deltaretrovirus, family Retroviridae, which is closely related to primate T-lymphotropic viruses including human T-cell lymphotropic virus 1 and 2 and simian T-cell lymphotropic virus.14,16 EBL is a contagious lymphoproliferative condition, and the most frequent neoplastic disease of cattle.14 The disease has been reported in almost every cattle raising country during the last century.12,14,16 However, to date, BLV has been successfully eradicated from over 20 countries, including several European countries, New Zealand, and Australia.6,14,20 Thus, it is mainly present in eastern Europe, the Americas, and some Asian and middle eastern countries.6,14 Most of these countries have reported continuous increases in BLV prevalence in beef and dairy cattle herds, although prevalence is usually higher in dairy farms.1,14,16 While BLV has been linked to breast cancer in women, the eventual causal relationship between this virus and breast cancer is still a matter of debate in the scientific community.2,5,8
BLV is harbored in circulating lymphocytes of infected cattle and can be transmitted horizontally and vertically; the former being the main form of transmission.6,13,14,20 As fresh blood, semen, saliva, milk, and nasal secretions are known sources of BLV proviral DNA, direct contact, iatrogenic procedures, blood-sucking insects, and natural breeding are potential transmission routes.6,14,20 Vertical transmission via transplacental infection or ingestion of colostrum/milk is thought to account for a small proportion of BLV infections.13,14 Intrauterine transmission has been reported to occur in 4–8% of calves born to BLV seropositive dams.6 Transmission through the ingestion of colostrum or milk from BLV-infected cows is possible since they both can contain provirus or free viral particles. However, colostrum and milk can also contain high titers of protective antibodies that could prevent infection in calves.12,13 Quantification of the risk of this transmission route is currently lacking.13
BLV is capable of infecting different immune cells, with a predilection for B-lymphocytes.16 Viral infection occurs after destabilization of the cell membrane by a transmembrane glycoprotein. Afterwards, the virus integrates into the host cell genome, interfering with gene expression and altering proliferative and apoptotic processes.14 These mechanisms are responsible for the different clinical features of the disease in cattle. On one hand, animals suffering from benign persistent lymphocytosis develop a massive proliferation of B-lymphocytes as a consequence of a blockage of their apoptosis along with an increase of proliferation.11 In contrast, neoplastic transformation is associated with inactivation of the tumor suppressor gene p53.14
Exposure to BLV may lead to four different outcomes: a) failure in establishing an infection, probably due to genetic resistance of the host and/or other factors; b) asymptomatic infection, which is characterized by detectable antibodies and no clinical or hematological abnormalities; c) persistent lymphocytosis with detectable antibody titers; and d) development of malignant lymphocytic neoplasia (lymphoma) in seropositive individuals.6,12,14 In the case described herein, the cow had evidence of persistent lymphocytosis in 2019, and while she was asymptomatic at that time, whether she concurrently had subclinical lymphoma was not determined. It is believed that the outcome of the infection may be influenced by the genetic constitution of the individual, its immune status, and the infective dose.6 The majority of infected cattle (70%) remain asymptomatic, while approximately 30% develop a benign lymphocytosis that persists for years, and it is not associated with the tumoral disease.6,14,17 The development of lymphoma occurs in 1–5% of BLV-infected individuals usually after a latency period of 1–8 years, regardless of the development of persistent lymphocytosis, and is considered the only symptomatic form of EBL.6,12,17 While lymphoma occurs regardless of the development of persistent lymphocytosis, it is estimated that approximately two thirds of the cows that developBLV-induced lymphoma previously undergo persistent lymphocytosis.11
BLV-induced lymphoma may be located in any organ, but the abomasum, kidney, heart, spinal cord, uterus, and lymph nodes are the most commonly affected ones.4,6 Therefore, the clinical presentation may vary depending on the organ or tissue involved.4,6 Overall, the most frequent clinical findings consist of inappetence, weight loss, drop in milk production, pallor, weakness, recumbency, etc.6,15 In the case presented here, although the heart was extensively affected, no signs or lesions compatible with congestive heart failure were detected, suggesting that extensive cardiac involvement may have occurred somewhat recently prior to clinical disease, or without significantly affecting cardiac function until a few weeks before euthanasia, when the cow became symptomatic. Additionally, because there was histologic evidence of neoplastic infiltration in the conduction system of the heart, as well as evidence of acute myocardial necrosis, we speculate that either one or both pathological findings could have precipitated the clinical deterioration in this case. Besides the heart and mesenteric lymph nodes, no other tissues were affected in this cow.
Macroscopically, lymphomas are yellowish, white to tan, bulging masses with a homogeneous aspect. Neoplastic tissue is usually firmer than normal lymphoid tissue, and it can be found surrounding bright yellow necrotic foci.3,6 In the heart, two gross presentations, consisting of nodular and diffuse forms, have been described.3 In the former, bulging lesions are located in the atrium and blend into the epicardial fat, thus making them hard to differentiate. Diffuse cardiac lesions are usually disseminated in the ventricular myocardium. Interestingly, our findings include both patterns since nodular lesions were present in the right atrium but there were also extensive lesions in the myocardium of the interventricular septum and left ventricular free wall.
Histological findings typically include dense cellular masses or diffusely infiltrative neoplasms composed of sheets of neoplastic round cells (B cells) that infiltrate, disrupt and/or replace the parenchyma of the affected organs.10 Neoplastic cells exhibit scarce cytoplasm and distinct cell borders, and large, irregularly round nuclei. The mitotic index is usually high.6
Clinically, differential diagnoses for the cardiac presentation include various conditions including bacterial infections (Histophilus somni, abscess, and bacterial endocarditis), parasitic diseases (i.e. sarcocystiosis, cysticercosis), traumatic reticulopericarditis, and primary cardiac tumors (rhabdomyoma, rhabdomyosarcoma, peripheral nerve sheath tumors [neurofibroma, schwannoma, neurinoma, neurofibroma], hemangioma, hemangiosarcoma, fibroma, fibrosarcoma, angiolipoma, angioleiomyoma, leiomyoma, leiomyosarcoma, adenomatoid tumor, hamartoma, mesothelioma, and myxoma).6,19 Enlargement of lymph nodes can be found in many other conditions, such as tuberculosis, paratuberculosis, caseous lymphadenitis, actinobacillosis, sporadic bovine leukosis (which is not caused by BLV), zygomycotic lymphadenitis, theileriosis, brucellosis, etc.6 Histologically, lymphoma is readily distinguishable from all these conditions, except for sporadic bovine leukosis, which usually occurs in young calves instead of adult cattle.
Antemortem diagnosis of BLV exposure and infection can be accomplished by serologic (ELISA, agar gel immunodiffusion) and molecular methods (PCR, qPCR). Lymphoma can be diagnosed by cytology and/or histopathology coupled with viral detection by PCR in postmortem specimens.6,14,20
Contributing Institution:
Plataforma de Investigación en Salud Animal
Instituto Nacional de Investigación Agropecuaria (INIA)
La Estanzuela, Uruguay
www.inia.uy
JPC Diagnoses:
- Myocardium: Lymphoma.
- Myocardium: Sarcocysts, few.
JPC Comment:
As the contributor details, aggressive efforts have led to the eradication of EBL in many countries; however, this fact belies the difficulty of eradication, largely due to the limited ability to identify “aleukemic” animals who are infected but subclinical.7 To that end, much of the current research surrounding EBL is focused on developing effective diagnostics that can be used to identify and remove asymptomatic animals from herds.
Among these new diagnostics are droplet digital PCR (ddPCR) assays that can be used to identify and quantify BLV provirus (viral DNA that has been integrated into the host genome) in animals with recent infection or low proviral load.7,15 ddPCR is a relatively recent nucleic acid quantification modality that has several benefits over PCR and quantitative PCR, including 1) the ability to provide quantitative counts without the need for a reference curve, and 2) the ability to provide reproducible quantitative data in unpurified samples that contain minute amounts of target DNA.7 Recent research shows that ddPCR can detect proviral DNA as early as 2 days post-infection, much earlier than the 13 days post infection with traditional PCR-based methods.7 ddPCR is also able to detect and quantify provirus in individual and bulk tank milk, an important diagnostic advantage allowing testing of easily accessible samples that have far lower levels of provirus and far higher levels of contaminates than required or allowed with legacy PCR techniques.7
The key innovation of the ddPCR technique is the fractioning of DNA samples into tens of thousands of individual droplets using an oil and water technique. Once the sample is fractionated, the PCR reaction is carried out in each individual droplet. Once the PCR reaction has terminated, the fluorescent properties of each individual droplet are analyzed in a method similar to flow cytometry, and the number of droplets that contain sample is used to quantify the original amount of proviral DNA.9
It is hoped that ddPCR quantification of proviral DNA levels in whole blood or milk may be a good indicator of disease and disease progression in the field.18 The quantification of proviral DNA can also inform herd management decisions as animals with low proviral loads are less effective virus transmitters and thus can be segregated, but need not necessarily be culled, to prevent disease transmission to naïve animals.7
The most recent research into BLV diagnostics has innovated on the ddPCR proviral assay. Previous research has identified an association between the bovine MHC-DRB3 gene and proviral load in certain species of cattle, with one allele associated with a high proviral load (a BLV susceptibility gene) and another allele associated with a low proviral load (a BLV resistant gene). This year, researchers developed a single-well assay that combines the ddPCR proviral quantitative assay with allele typing, including identification of homo- and heterozygotes, to provide a more holistic view of which animals are at risk of spreading disease.15 These diagnostic innovations could soon provide a pathway for economically viable eradication efforts that minimize culling, even in areas of high disease prevalence.
This case presents a classic demographic, clinical, gross, and histologic case of BLV-induced lymphoma. In a heart with this gross appearance, lymphoma should be at the top of the differential list, though neurofibroma and granulomatous disease can’t be definitely ruled out prior to histologic evaluation. Conference participants discussed the curious subgross appearance of the examined tissue section, which contains a large confluent, well-demarcated area of pallor reminiscent of an infarct; however, histologically, while neoplastic cells in this area are often necrotic, the cardiomyocytes appear viable. No conclusions were reached as to the origin of this unique appearance.
The scattered sarcocysts are a common, incidental finding in cattle and are most commonly tissue cysts of Sarcocystis cruzi.
References:
- Bartlett PC, Ruggiero VJ, Hutchinson HC, et al. Current developments in the epidemiology and control of Enzootic Bovine Leukosis as caused by bovine leukemia virus. Pathogens. 2020;9(12): 1058.
- Buehring GC, Shen HM, Schwartz DA, Lawson JS. Bovine leukemia virus linked to breast cancer in Australian women and identified before breast cancer development. PLoS One. 2017;12(6):
- Buergelt C, Clark E, del Piero F. Bovine Pathology: A Text and Color Atlas. CABI International;2018:285.
- Burton A, Nydam D, Long E, Divers T. Signalment and clinical complaints initiating hospital admission, methods of diagnosis, and pathological findings associated with bovine lymphoma (112 cases). J Vet Intern Med. 2010; 24:960-964.
- Ceriani MC, Lendez PA, Martinez Cuesta L, et al. Bovine Leukemia Virus Presence in Breast Tissue of Argentinian Females and Its Association With Cell Proliferation and Prognosis Markers. Multidiscip Cancer Investig. 2018; 2(4):16–24.
- Constable P, Hinchcliff K, Done S, Grünberg W. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th ed. Saunders Elsevier; 2017:785–794.
- DeBrun ML, Cosme B, Petersen M, et al. Development of a droplet digital PCR assay for quantification of the proviral load of bovine leukemia virus. J Vet Diagn Invest. 2022;34(3):439-447.
- Delarmelina E, Buzelin MA, de Souza BS, et al. High positivity values for bovine leukemia virus in human breast cancer cases from Minas Gerais, Brazil. PLoS One. 2020;15(10):e0239745.
- Hindson BJ, Ness KD, Masquelier DA, et al. High-throughput droplet digital PCR system for absolute quantification of DNA copy number. Anal Chem. 2011;83(22):8604-8610.
- Hishamnuri WNAD, Nakagun S, Maezawa M, et al. Disseminated thymic B-cell lymphoma in a Holstein heifer. J Vet Diagn Invest. 2019;31(6):852–855.
- Juliarena MA, Gutierrez SE, Ceriani C. Determination of proviral load in bovine leukemia virus–infected cattle with and without lymphocytosis. Am J Vet Res. 2007;68(11):1220–1225.
- Juliarena MA, Barrios CN, Lützelschwab CM, Esteban EN, Gutiérrez SE. Bovine leukemia virus: Current perspectives. Virus Adapt Treat. 2017;9:13–26.
- Kuczewski A, Orsel K, Barkema HW, Mason S, Erskine R, van der Meer F. Invited review: Bovine leukemia virus—Transmission, control, and eradication. J Dairy Sci. 2021;104(6):6358–6375.
- Marawan MA, Alouffi A, El Tokhy S, et al. Bovine leukaemia virus: Current epidemiological circumstance and future prospective. Viruses. 2021;13(11):1–24.
- Notsu K, El Daous H, Mitoma S, Wu X, Norimine J, Sekiguchi S. Identifying pathogen and allele type simultaneously in a single well using droplet digital PCR. mSphere. 2023;8(1):e0049322.
- Polat M, Takeshima SN, Aida Y. Epidemiology and genetic diversity of bovine leukemia virus. Virol J. 2017;14(1):1–16.
- Schwartz I, Lévy D. Pathobiology of bovine leukemia virus. Vet Res. 1994; 25(6):521–536.
- Smith B, Van Metre D, Pusterla N. Large Animal Internal Medicine. Elsevier Mosby; 2020:1874.
- Une Y, Shirota K, Nomura Y. Cardiac angioleiomyoma in 44 cattle in japan (1982-2009). Vet Pathol. 2010;47(5): 923–930.
- World Organisation for Animal Health (OIE). Enootic Bovine Leukosis. In: OIE Terrestrial manual. 8th ed. OIE;2018:1-12.