WSC 2023-2024, Conference 14, Case 3
Signalment:
19-year-old, Polo mare (Equus caballus)
History:
This 19-year-old Polo mare presented with a recent history of coughing and exercise intolerance.
Gross Pathology:
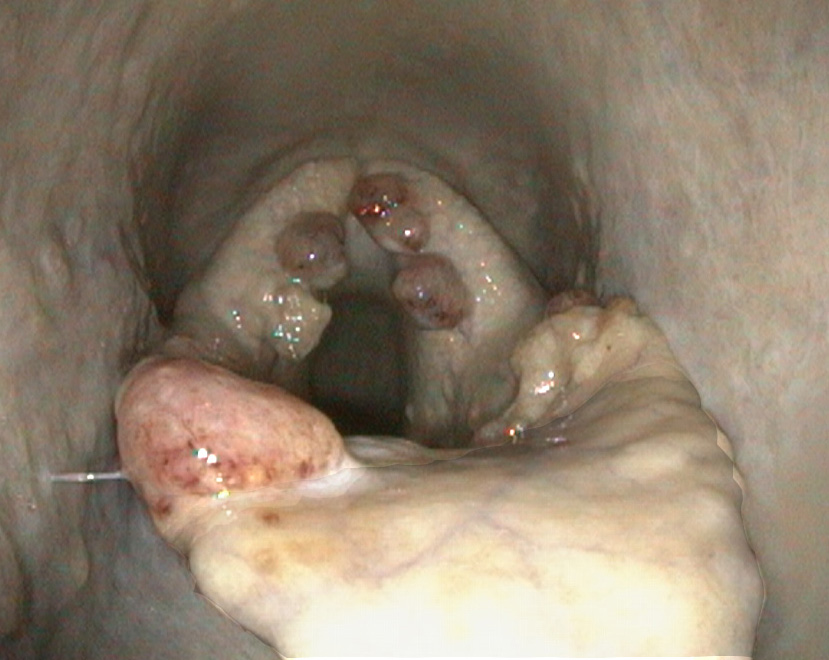
On endoscopy, several irregular, broad, pedunculated, polypoid lesions were detected at the laryngeal aditus and aryepiglottic folds. The masses were mottled cream to red-brown and slightly granular.
Microscopic Description:
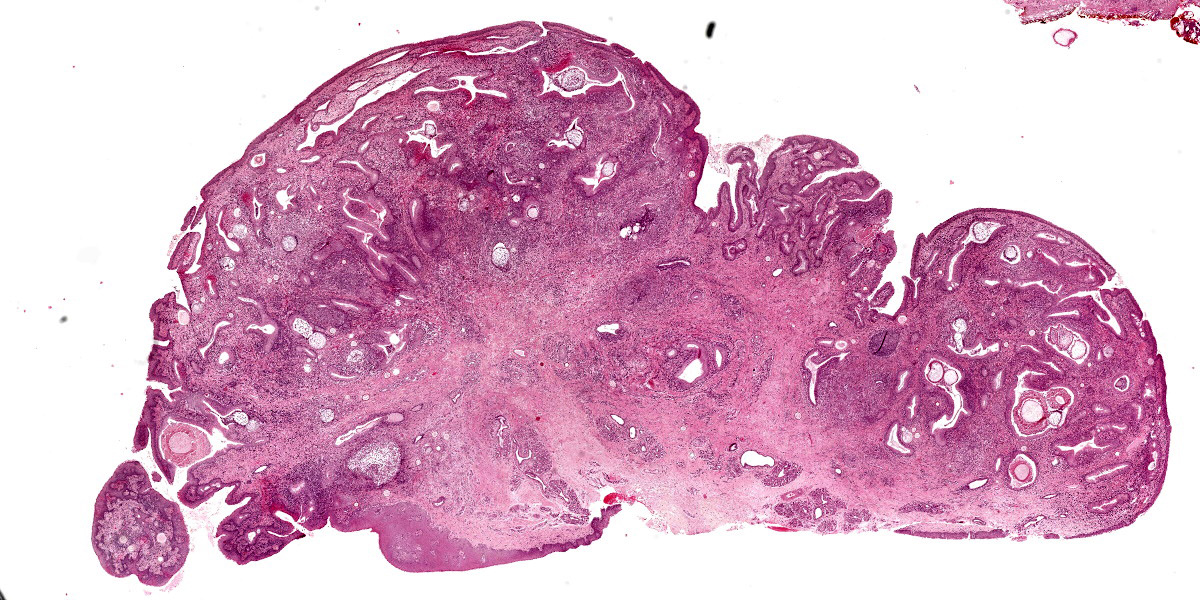
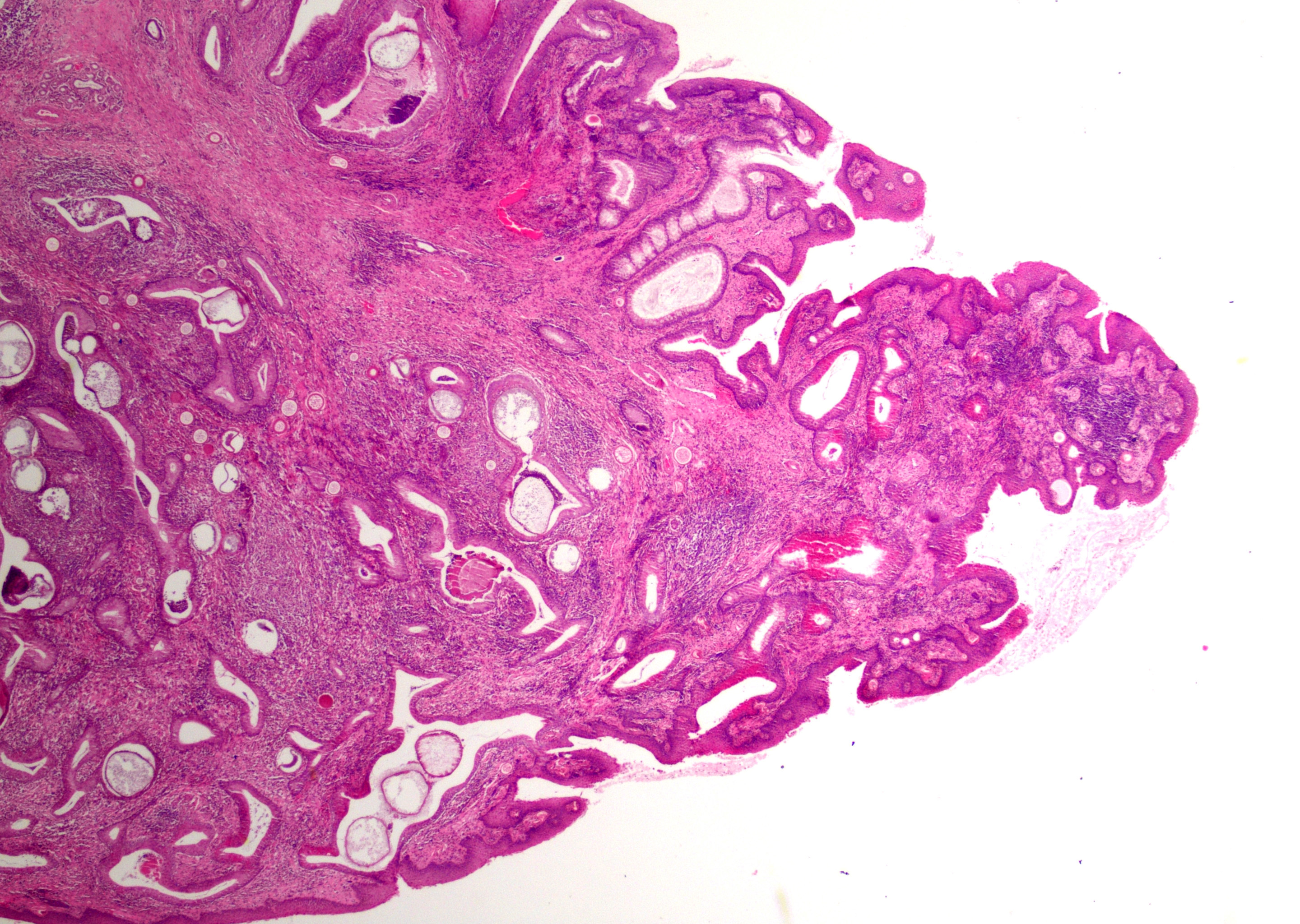
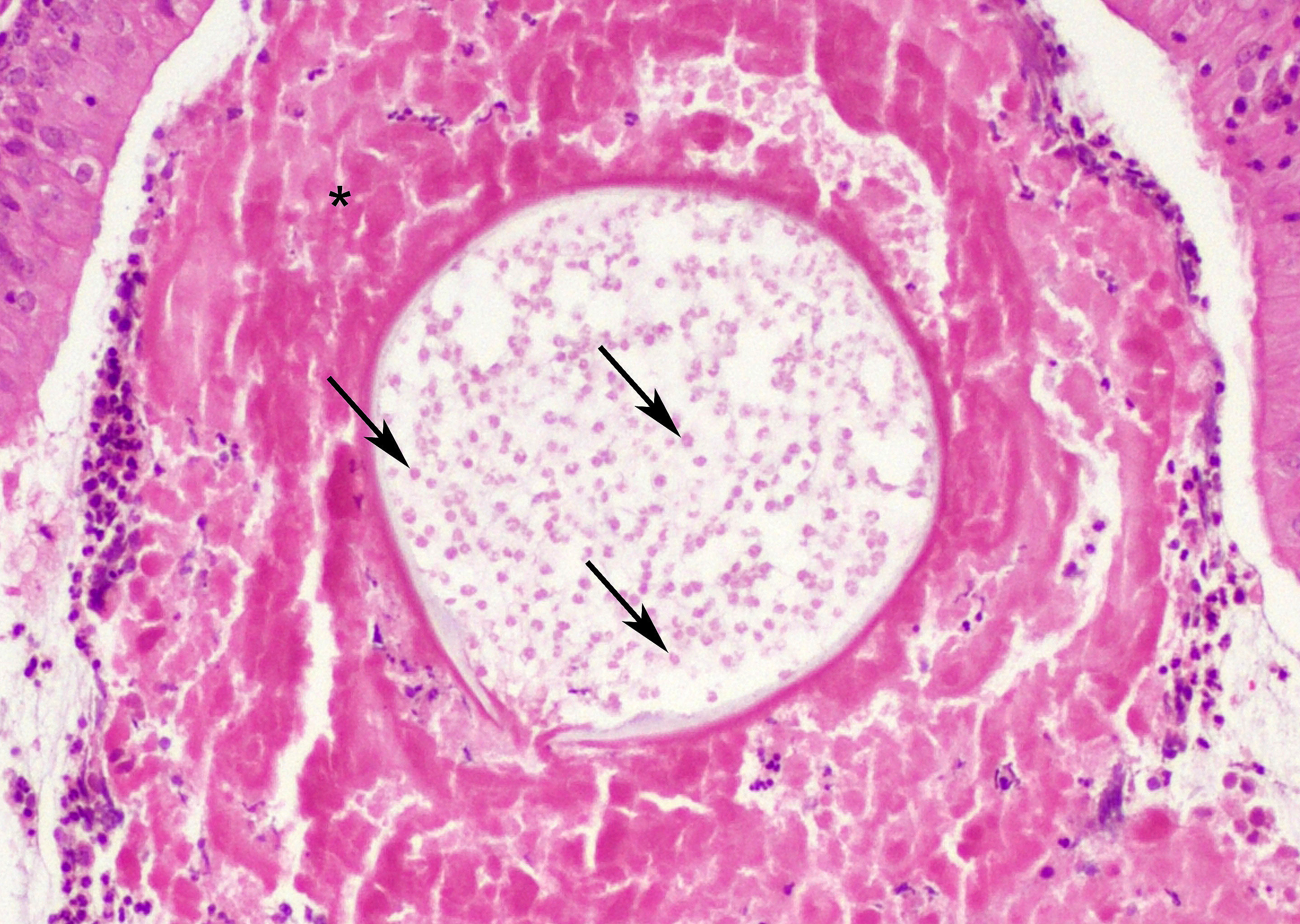
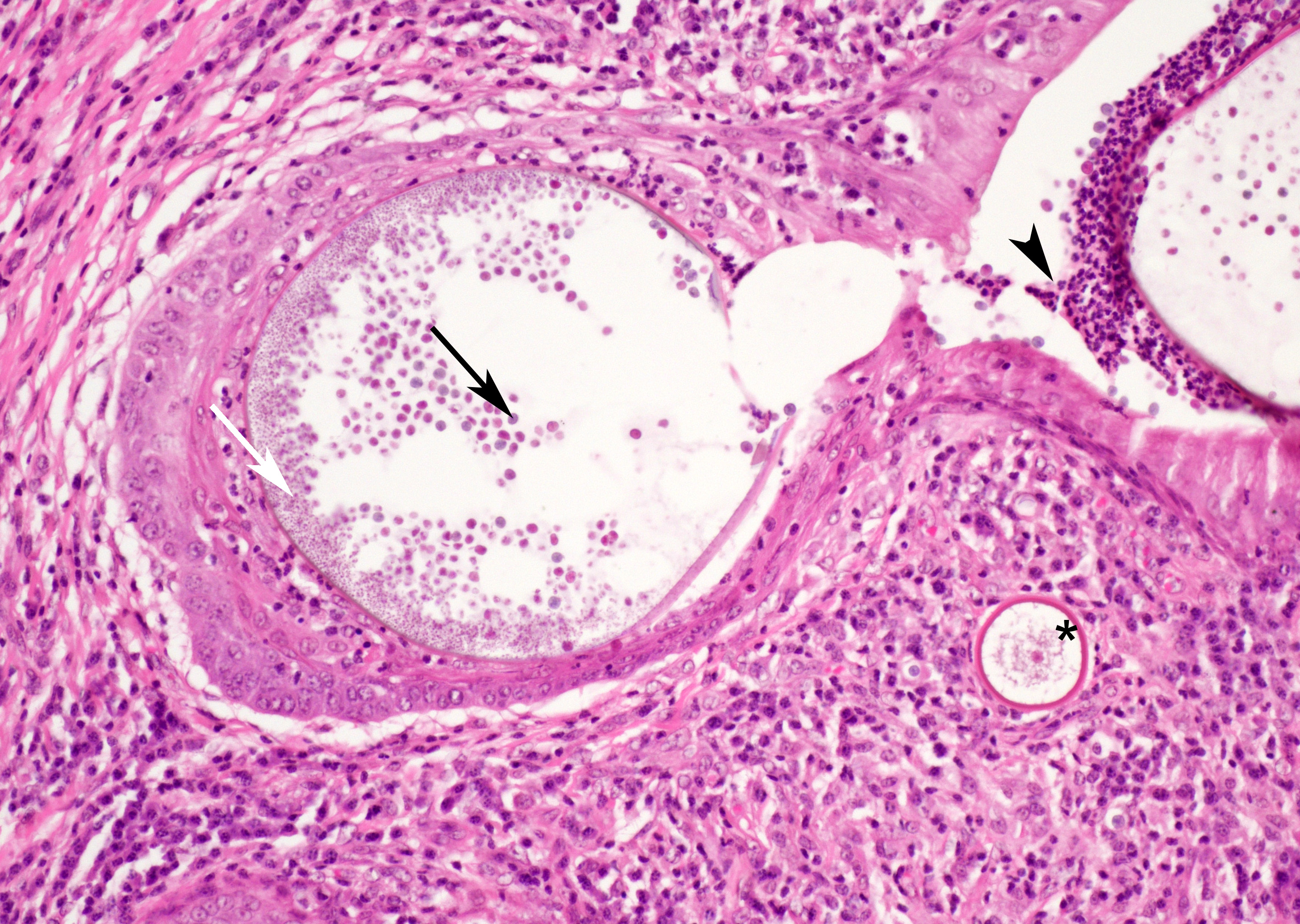
At low magnification, raised polypoid proliferations of loose fibrovascular tissue covered by an intact, mildly hyperplastic, non-keratinizing stratified squamous epithelium expand the mucosa and submucosa. Numerous spherical structures of varying size are randomly distributed throughout the lamina propria and submucosa, and are occasionally present within the laryngeal epithelium. These spherical structures represent sporangia in different stages of development. Sporangia are also present within submucosal glands that are dilated and are sometimes surrounded by deposits of amorphous eosinophilic material.
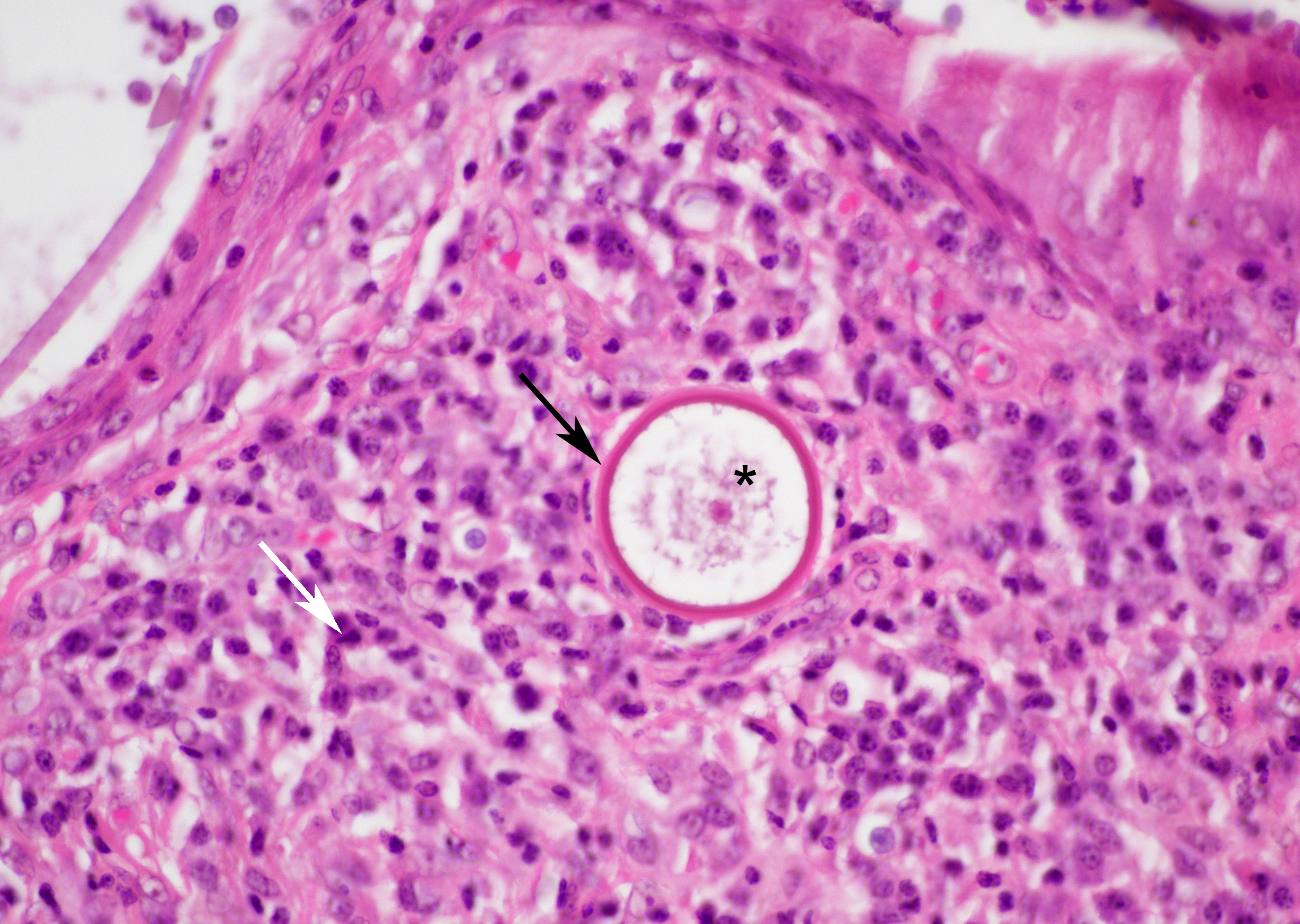
Small juvenile, immature sporangia (trophocytes) measure 10?100 μm, have a thick unilamellar, eosinophilic wall, and a single central round nucleus surrounded by granular amphophilic cytoplasm. Intermediate sporangia are characterized by the loss of the central single nucleus, a size of up to 300 μm, and the presence of numerous punctate eosinophilic granules. With progressing maturation, nuclei become more discrete 1?4 μm ovoid structures (prophase nuclei) and intermediate sporangia exhibit a mixture of punctate granules and small eosinophilic ovoid structures.4
Mature sporangia are spherical and 100?400 μm in diameter with a bilamellar, outer eosinophilic and inner amphophilic wall. The mature endospores are 10 μm, eosinophilic to basophilic, ovoid to round structures with a cell wall and single nucleus. Often a centripetal or centrifugal zone of small round eosinophilic structures (germinative zone) and mature endospores is present within the mature sporangia. Multifocally, the mature sporangia are ruptured or release endospores through their operculum (pore). Rupture of sporangia is accompanied by infiltration of large numbers of neutrophils and fewer macrophages.
Moderate numbers of lymphocytes and plasma cells and fewer neutrophils are multifocally present within the stroma of the lamina propria and the submucosa. Multifocally, small numbers of neutrophils migrate into the overlying mucosal epithelium (exocytosis).
A thin layer of coagulative necrosis (cautery artefact) is present around the margins and is often associated with mild multifocal oedema and haemorrhage. In some of the sections examined, sporangia are detected very close to the areas of cautery.
Contributor’s Morphologic Diagnosis:
Laryngitis, proliferative, lymphocytic, pla-smacytic, neutrophilic, and histiocytic, multifocal to coalescing, moderate, chronic, with intralesional juvenile and mature sporangia consistent with Rhinosporidium seeberi.
Contributor’s Comment:
Rhinosporidium seeberi is a eukaryotic, hydrophilic organism from the Mesomycetozoa clade (DRIP clade) that also includes several fish pathogens such as Dermocystidium spp.11,18 Its exact phylogenetic classification is unclear, with some discussion as to whether it should be grouped differently. The organism is generally found in stagnant waters and is the causative agent of rhinosporidiosis in humans.11,17 In addition, rhinosporidiosis has been described in multiple other species, including cats, horses, cattle, buffaloes, dogs, mules, swans, and wild fowls.3,14,17 Rhinosporidiosis is endemic in India, Sri-Lanka, in parts of the Americas, and Africa.12,14,17 In horses, in addition to endemic areas, cases have also been recorded in countries traditionally free of rhinosporidiosis. Those cases have mostly been restricted to imported animals, such as four polo ponies that were imported from Argentina to the United Kingdom.4,12,17 However, the potential for the disease to inhabit traditionally non-endemic areas was highlighted by a recent report from Europe. In that case, a Belgian warmblood horse, born in Belgium, that had never left the country, was diagnosed with rhinosporidiosis.15 The source of infection remained unclear; however, as this organism is not contagious, the case suggests that the organism was present in the environment in Belgium.16
Due to difficulties with in vitro culturing of this organism, the life cycle of Rhinosporidium seeberi is still not fully understood.18 It is believed that the infective stage is the mature endospore which enters the host through damaged epithelium of mucous membranes, mostly of the nose.10,18 In the host tissue the endospore develops into a juvenile sporangium (trophocyte) with a single central nucleus. Subsequent reproduction takes place in intermediate sporangia via sole synchronized nuclear division. Cytokinesis occurs only once shortly before the formation of the mature sporangium. 9,13 Furthermore, it is suggested that mature sporangia are capable of additional “de novo” production of mature, cell-walled endospores.9
Based on the finding that only a few humans out of the many bathing in contaminated water become infected with Rhinosporidium seeberi, an individual host susceptibility has been suggested. The susceptibility is assumed to correlated to the presence of certain blood groups, but this relation has not be confirmed.2
Rhinosporidiosis most often localises in mucous membranes of the head region, with the nostrils and conjunctivae most commonly affected.1,10 In humans, infection of the throat, eye, ear, oropharynx, larynx, trachea, bronchi, and genitourinary tract are also described.7,16 Infected tissues develop single to multiple, pink, multilobular, broad-based, granulomatous, pedunculated, non-infiltrative masses. The affected mucosa is often red with stippled, pinpoint white granules (mature sporangia) that give the mass a mulberry-like or strawberry-like appearance.5 The masses are slow growing and generally painless; however, irritation due to the presence of the masses can lead to sneezing, discharge, and when obstructing airways, may result in respiratory distress.5,8
In this horse, multiple polypoid masses were detected around the laryngeal aditus and at the aryepiglottic folds, resulting in coughing and exercise intolerance. The localisation in the laryngeal mucosa is rather unusual in horses. To our knowledge, two cases of equine laryngeal rhinosporidiosis have been described so far. In both of these cases, the masses were centred around the larynx, were surgical excised, and recurred.4,15 So far, half a year after surgical excision, no recurrence was observed in this horse.
Rhinosporidiosis remains an uncommon infection that can be diagnostically challenging. The diagnosis of equine rhinosporidiosis is based on clinical history, characteristic macroscopic and histopathologic appearance, and potentially, confirmatory polymerase chain reaction.4,12,15 Treatment of choice is surgical excision with broad, clear margins and electrocauterization or cryotherapy of adjacent tissues4,5,15 In humans, additional administration of dapsone is used to prevent maturation.10 Recurrence was reported to occur in 11% of human cases and is due to spreading of mature endospores.10,12 In addition, due to a long incubation period reported to be at least 10 months in one pony, clinically inapparent animals may be transported from endemic areas and might shed the organism.12 Given the long incubation period, increased international movement of animals, and climate change, rhinosporidiosis has the potential to be an emerging disease and to become endemic in areas that have traditionally been free of rhinosporidiosis.
Contributing Institution:
Department of Veterinary Medicine
The Queen's Veterinary School Hospital
University of Cambridge.
Cambridge CB3 0ES, UK.
https://www.vet.cam.ac.uk
JPC Diagnosis:
Larynx: Laryngitis, proliferative, chronic-active, diffuse, moderate, with numerous juvenile and mature sporangia and endospores.
JPC Comment:
The contributor provides an excellent, thorough summary of Rhinosporidium seeberi infection, a classic disease with a classic, virtually unmistakable histological appearance. As the contributor notes, the laryngeal location is uncommon; the more typical presentation of rhinosporidiosis is a soft, pink unilateral fleshy polyp, usually in the nose of a dog.6
While the gross appearance of R. seeberi may be confused for a variety of inflammatory or neoplastic conditions, particularly when found in an unusual location, once under the microscope, R. seeberi sporangia are not histologic wallflowers, but are large, striking, and distinctive. The juvenile sporangia are 15-75µm in diameter, have a unilamellar, PAS-positive wall, and a single nucleus.6 The real stars, however, are the mature sporangia, which can grow to an astonishing 100-400 µm, have a PAS-positive bilamellar wall, and contain myriad 5-10 µm endospores. The presence of these eye-catching sporangia in the histologic section of a nasal polyp is essentially pathognomonic for rhinosporidiosis.
R. seeberi belongs to a class of organisms aptly termed endosporulators. Other endosporulators of veterinary importance include Coccidioides immitis, Coccidioides posadasii, Prototheca sp., Chlorella sp., and Batrachochytrium dendrobatidis. Of these, only Coccidioides is of a size and morphology that could be confused with R. seeberi. Immature Coccidioides spherules, the analogue to the R. seeberi sporangia, are approximately 10-20 µm in diameter and grow into mature spherules of up to 200 µm in diameter filled with numerous 2-5µm endospores.6
Participants struggled with tissue identification with this slide, with most defaulting to nasal mucosa based on the typical location. Conference discussion focused largely on the morphologic diagnosis and how to capture the significant, mixed inflammatory component of the lesion. Some participant felt strongly that the morphologic diagnosis should emphasize the extensive lymphohistiocytic and plasma-cytic character of the lesion, while others argued that the significant neutrophilic infiltrate should also be included. In the end, participants decided that “chronic-active” implied the existnence of both a neutrophilic (active) component and a lymphohistiocytic component with marked hyperplasia of the mucosa (a chronic change) and preferred the pared down diagnosis given above.
References:
- Allison RW, Ramachandran A. What is your diagnosis? Ulcerative nasal lesion in a Quarter Horse. Vet Clin Path. 2015; 44: 455-456.
- Arseculeratne SN. Recent advances in rhinosporidiosis and Rhinosporidium seeberi. Indian J Med Microbiol. 2002;20: 119-131.
- Berrocal A, López A. Nasal rhino-sporidiosis in a mule. Can Vet J. 2007;48: 305-306.
- Burgess HJ, Lockerbie BP, Czerwinski S, Scott M. Equine laryngeal rhino-sporidiosis in western Canada. J Vet Diagn Invest. 2012;24(4):77-780.
- Caniatti M, Roccabianca P, Scanziani E, et al. Nasal rhinosporidiosis in dogs: four cases from Europe and a review of the literature. Vet Rec. 1998;142:334-338.
- Caswell JL, Williams K. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. 2016; Elsevier:579-584.
- Costa EF, Pinto LM, Campos MAG, Gomes TM, et al. Partial regression of large anterior scleral staphyloma secon-dary to rhinosporidiosis after corneo-scleral graft - a case report. BMC Ophthalmology. 2018;18:61.
- Das S, Kashyap B, Barua M, et al. Nasal rhinosporidiosis in humans: new interp-retations and a review of the literature of this enigmatic disease. Medical Mycol. 2011;49:311-315.
- Delfino D, Mendoza L, Vilela R. Rhinosporidium seeberi nuclear cycle activities using confocal microscopy. J Parasitol. 2016;102(1):60-68.
- Harissi-Dagher M, Robillard N, Corriveau C, Mabon M, et al. Histopathologically confirmed ocular rhinosporidiosis in two Canadians. Can J Ophthalmol. 2006;41: 226-229.
- Herr RA, Ajello L, Taylor JW, Arsecul-eratne SN, Mendoza L. Phylogenetic analysis of Rhinosporidium seeberi’s 18S small-subunit ribosomal DNA groups this pathogen among mem-bers of the Protoctistan Mesomycetozoa Clade. J Clin Microbiol. 1999;37(9):2750-2754.
- Leeming G, Smith KC, Bestbier ME, Barrelet A, Kipar A. Equine rhinosporidiosis in United Kingdom. Emerg Infect Dis. 2007;13(9):1377-1379.
- Mendoza L, Vilela R. Presumptive synch-ronized nuclear divisions without cyto-kinesis in the Rhinosporidium seeberi parasitic life cycle. Microbiology. 2013; 159:1545-1551.
- Moses JS, Balachandran C. Rhino-sporidiosis in bovines of Kanyakumari district, Tamil Nadu, India. Myco-pathologia. 1987;100:23-26.
- Nollet H, Vercauteren G, Martens A, et al. Laryngeal rhinosporidiosis in a Belgian warmblood horse. Zoonoses Public Health. 2008;55:274-278.
- Putthia H, Manjunatha BS, Astekar M, Taufiq S. Palatal rhinosporidiosis: an unusual case report and review of the literature. J Korean Assoc Oral Maxillofac Surg. 2018;44:293-297.
- Sudasinghe T, Rajapakse RPVJ, Perera NAND, et al. The regional sero-epidem-iology of rhinosporidiosis in Sri Lankan humans and animals. Acta Tropica. 2011;120:72-81.
- Vilela R, Mendoza L. The taxonomy and phylogenetics of the human and animal pathogen Rhinosporidium seeberi: A critical review. Rev Iberoam Micol. 2012;29(4):185-199.