CASE 2: N449-19B (4154915-00)
Signalment:
11-week-old, male, Hungarian Vizsla, canine
History:
Died one day following acute onset pyrexia, vomiting, diarrhoea and marked jaundice (icterus). An abdominal scan revealed free-fluid in the peritoneal cavity and bilaterally in the peri-renal regions. The puppy had been given its first vaccination against leptospirosis (L. canicola and L. icterohaemorrhagiae) and canine parvovirus-2, 2 weeks previously.
Gross Pathology:
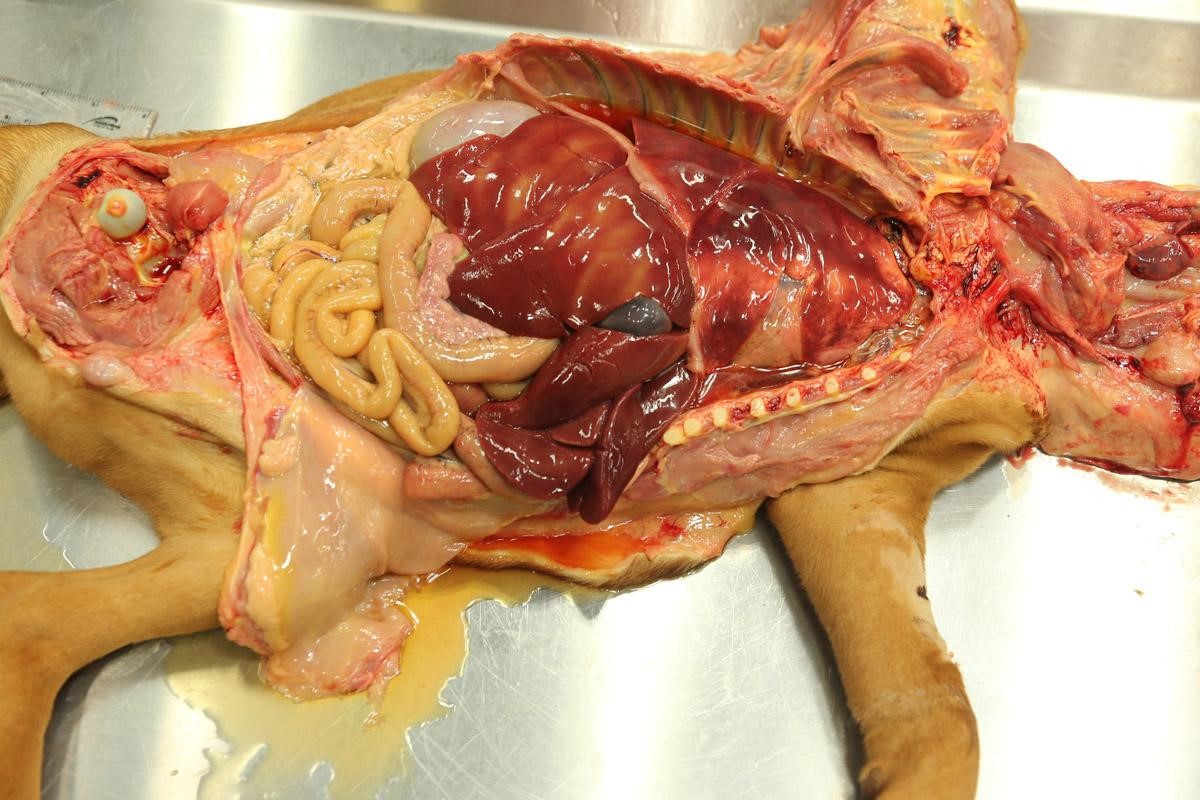
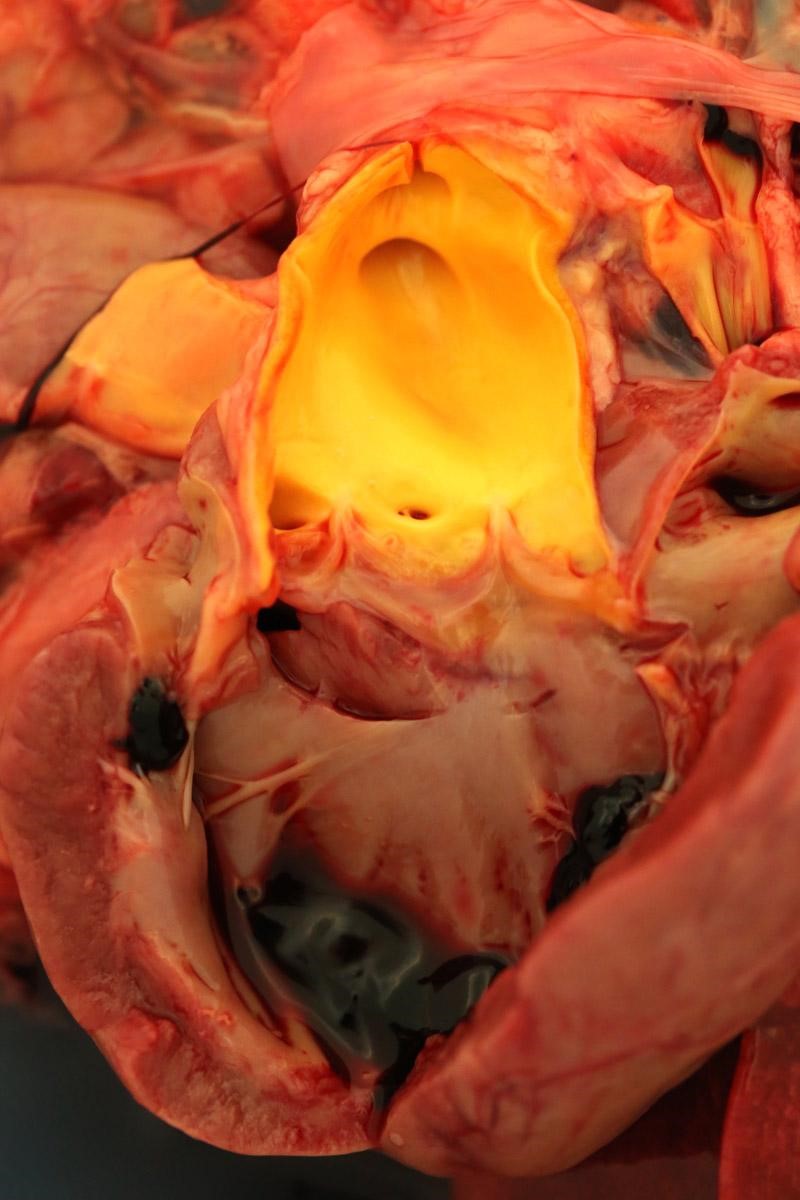
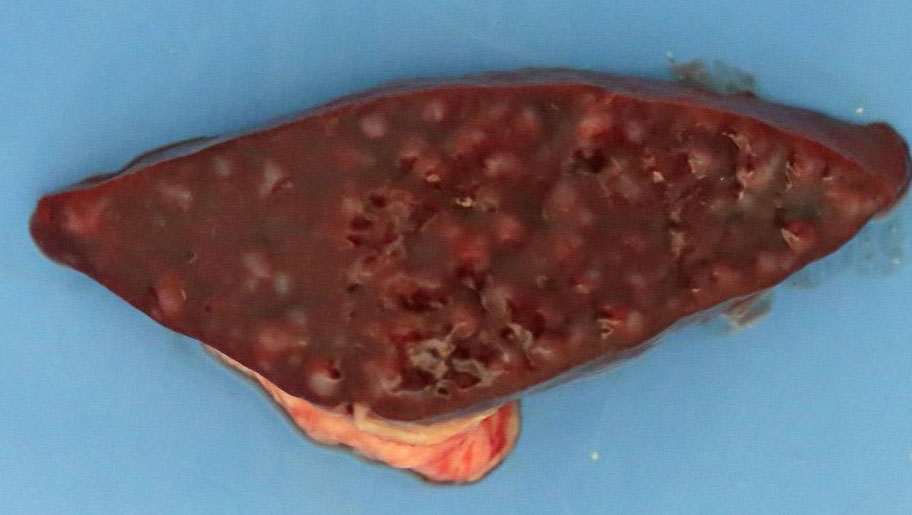
Weighed 8.2kg. In good body condition with diffusely yellow mucous membranes, serosal surfaces and sclera. 20ml and 5ml of yellow translucent fluid found in thoracic and pericardial cavities, respectively. Bilaterally the lungs are wet with dark-red mottling. Diffuse yellow staining of pulmonary arterial and aortic endothelia. 30-40ml of yellow translucent fluid in peritoneal cavity. No food in stomach. Gelatinous yellow mucus in small intestinal lumen. Liver diffusely soft featuring pale yellow regions and has a wet glistening capsular surface. Feces scant, mucoid and yellow. Splenomegaly with prominent white pulp on cut section. Sludge-like dark green bile distends the gall bladder. Kidneys diffusely pale and tinged yellow with petechiation of capsule. Very small quantity of normal-appearing urine in bladder.
Laboratory results:
Raised alkaline phosphatase, alanine transaminase, total bilirubin and bile acids.
Pathogenic Leptospira sp. confirmed by quantitative PCR using primers detecting lipoprotein LipL32 and Taqman probe. Further characterized as L. icterohaemorrhagiae by PCR using SYBR green probe to identify species by targeting secY.
Microscopic description:
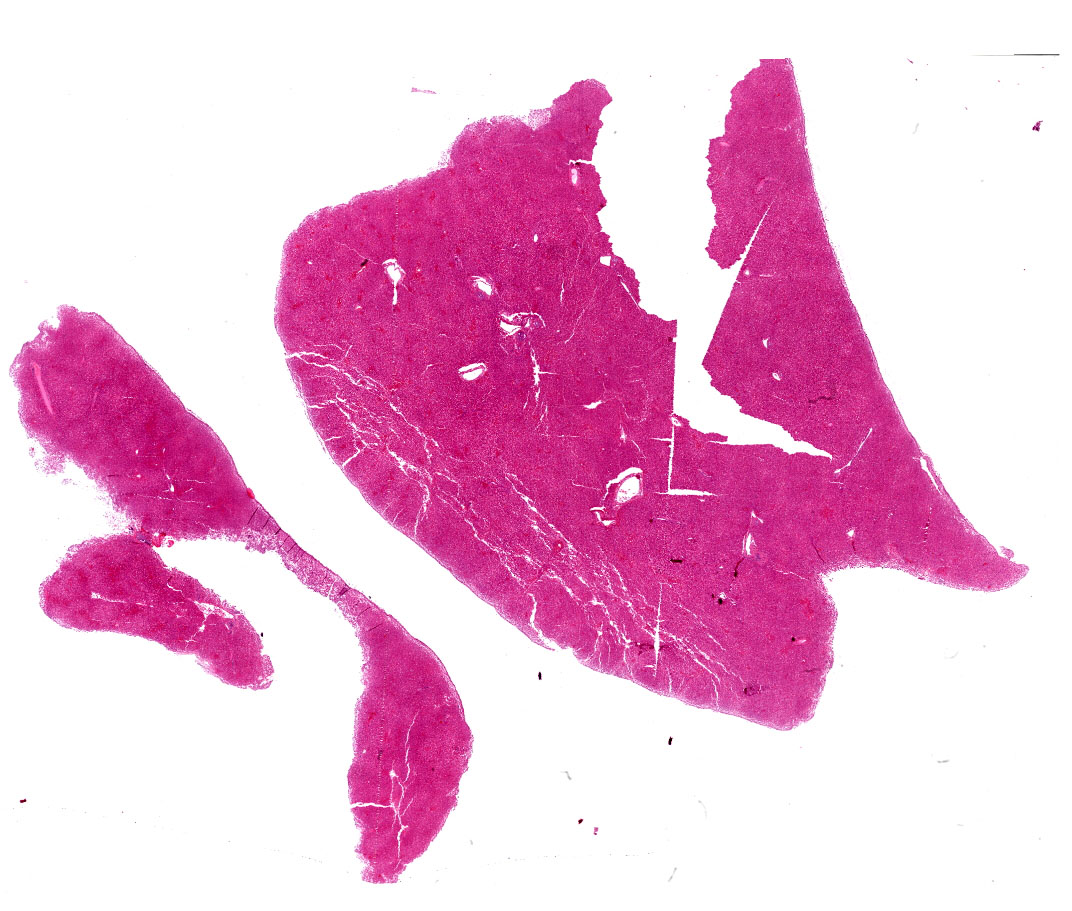
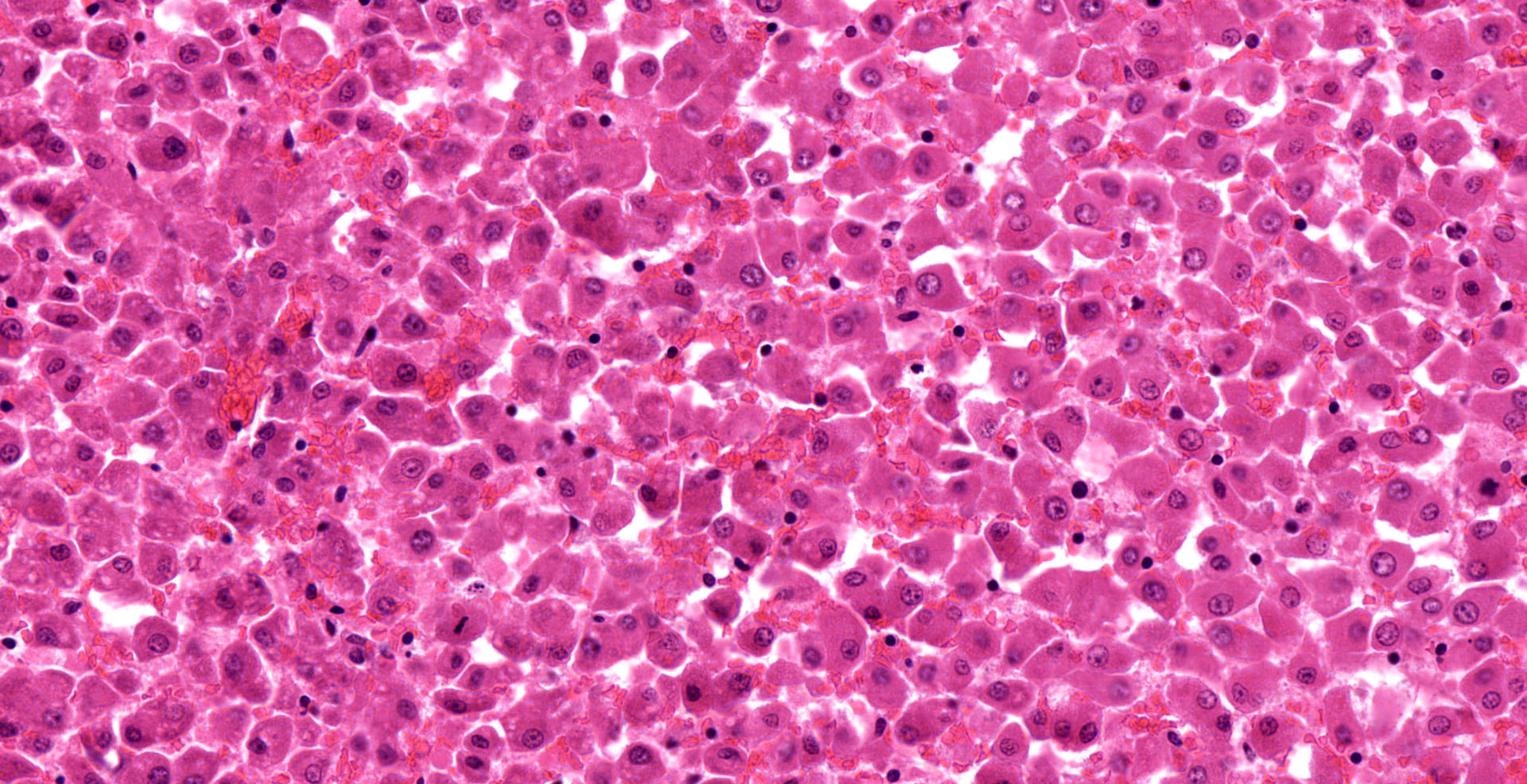
Liver: diffuse hepatocyte dissociation (loss of inter-cellular connectivity) with approximately 1-2 hepatocytes per HPF exhibiting mitotic activity (hepatic proliferation). Individualized hepatocytes have a more rounded outline and often feature a pyknotic nucleus. Sinusoidal channels are multifocally congested with widespread disruption of their endothelial lining. Diffusely Kupffer cells are prominent (activated). Occasional isolated both necrotic and binucleate hepatocytes noted.
Hyperplasia of periarteriolar lymphoid sheaths noted in spleen along with occasional necrosis of lymphocytes. Glomerular congestion with occasional proteinaceous cast in collecting ducts. Diffuse protein-rich pulmonary oedema with attendant diffuse congestion of alveolar walls and abundant macrophages in alveolar lumens.
Contributor's morphologic diagnosis:
Hepatocyte dissociation, diffuse, marked with occasional individual cell necrosis and attempted regeneration
Contributor's comment:
A diagnosis of leptospirosis was reached based on: signalment, clinical signs, characteristic, microscopically visible hepatic lesions and positive PCR. Acute fatal leptospirosis in dogs presents as fulminant septicemia/hepatic disease largely affecting young animals.3,9 The often-subtle hepatic changes evident microscopically may present a diagnostic challenge and in many cases the puppy dies prior to the establishment of detectable antibody. In the current case, it would appear that the initial step in the puppy's vaccination protocol did not provide sufficient immunological protection.
Canine leptospirosis may occur as peracute, acute, subacute, or chronic disease.3,9 The peracute/acute form as described here typically results from infection with serovar Icterohaemorrhagiae, although clinical disease caused by this serovar (and serovar Canicola) are now relatively rare due to widespread vaccination.10,11 Widespread hemorrhage may be a gross postmortem feature: mucosal hemorrhages, hematemesis, epistaxis, and melena.3,9 The marked jaundice in this case occurred in the absence of either hemolysis or post-hepatic biliary obstruction. The microscopically visible widespread hepatocyte dissociation described is characteristic and is considered the result of leptospires disrupting inter-cellular tight junctions between hepatocytes. In a hamster infection model leptospires infiltrated the space of Disse, migrated between hepatocytes, detached the intercellular junctions and disrupted bile canaliculi: this disruption coincided with the elevation of conjugated bilirubin, aspartate transaminase and alkaline phosphatase levels in serum.7 Leptospires have a characteristic spiral shape and corkscrew motion, features considered to facilitate tissue invasion and their ability to bore through highly viscous gel-like media, such as connective tissues, which inhibit the motility of most other bacteria. Bacterial-shaped grooves observed on hepatocyte surfaces are thought to reflect this boring activity which ultimately result in cell from cell detachment.1 Thus, leptospires are considered invasive but not facultative intracellular pathogens: they can invade but also rapidly exit cells so as to avoid the attention of host leukocytes. How they achieve this high-speed cell translocation remains to be ellucidated.1 Given a key function of inter-hepatocyte tight junctions is to seal bile canaliculi, their breakdown results in disruption to canalicular structure and thus to bile flow out of the liver: intra-hepatic cholestasis is the consequence. This increased paracellular permeability causes regurgitation of bile constituents into plasma and the attendant hyperbilirubinemia.2,7
The fragility of leptospires means they are most effectively transmitted by direct contact. Following penetration of mucosal surfaces or water-softened skin or ingestion of contaminated water, organisms enter the host bloodstream where leptospiremia can last for up to 7 days.4,10 This persistent leptospiremia would account for the splenic white pulp hyperplasia/activation in this case. In the bloodstream, leptospires evade the host immune response by binding inhibitors of complement on their surface.10 They invade and multiply particularly well in organs including liver, kidneys, lungs, placenta, and udder. Agglutinating and opsonizing antibody develops at approximately 6 days and clears the organism from most organs except immunologically privileged sites such as the kidney and eye.3
Classically, hosts for leptospirosis have been subdivided into 'primary/maintenance' and 'incidental/accidental'.3 Dogs are the primary host for Leptospira Canicola and incidental hosts for Leptospira Icterohaemorrhagiae where the primary host is the rat. In dogs that survive acute leptospirosis, the burden of disease shifts from the liver to the kidneys.3 This is typical of infections caused by serovar Canicola and by 'incidental' serovars infecting the dog such as Grippotyphosa and Pomona. Post-leptospiremic localization of organisms in the kidneys is associated with interstitial nephritis. The organism replicates and persists in the convoluted tubular epithelium, even in the presence of neutralizing antibodies. Acute impairment of renal function may result from decreased glomerular filtration caused by swelling that impairs renal perfusion, along with tubular epithelial swelling and necrosis.3 Renal function in some dogs that survive acute infections may return to normal within several weeks, or chronic renal failure may develop. Typical subacute renal changes consist of moderate to severe lymphoplasmacytic and neutrophilic tubulointerstitial nephritis, with tubular degeneration, necrosis, and mineralization. In chronic cases interstitial fibrosis and tubular atrophy dominate.3 Dogs that recover can excrete organisms in their urine intermittently for several months. The WHO consider leptospirosis a neglected but significant zoonosis with global reach.4,7,10,12
Dogs with leptospirosis may present with respiratory signs. A similar leptospiral pulmonary hemorrhage syndrome (LPHS) is recognized in human leptospirosis with mortality rates of greater than 50%. The pathogenesis of this syndrome remains to be elucidated but would appear to be mediated by systemic inflammatory and/or immune mechanisms.10
Widespread vaccination of dogs against serovars Canicola and Icterohaemorrhagiae over many decades has resulted in epidemiological shifts to infection with serovars such as Grippotyphosa and Pomona. This has necessitated the modification of vaccines to provide protection against these and possibly other serovars depending on geographical location.10,11
The taxonomy of Leptospira remains complex and is based on: (i) serovar - reflecting differences in response of host antibody to the organism's lipopolysaccharide structure; (ii) serogroup - groups of antigenically related serovars; and (iii) genomospecies -organisms grouped based on genomic similarity.4,11
Contributing Institution:
Veterinary Sciences Centre
School of Veterinary Medicine
University College Dublin
Belfield, Dublin 4, Ireland
JPC diagnosis:
Liver: Hepatocellular dissociation, diffuse, severe, with random individualized hepatocellular degeneration and necrosis.
JPC comment:
The contributor provides an excellent summary of this interesting disease. This case may be consistent with previous reports of chronic hepatitis caused by Leptospira spp, without renal involvement, in kenneled Foxhounds and a colony of Beagles in England, and a second study of 10 dogs of a variety of breeds. The case selection criteria included dogs with granulomatous chronic hepatitis, a definition provided by the World Small Animal Veterinary Association (WSAVA). These dogs were asymptomatic but had elevated hepatocellular enzymes (ALT) and cholestatic enzymes (ALP), and many had elevated bilirubin, bile acids, and cholesterol. Using fluorescence in situ hybridization (FISH) on liver biopsies, clusters of leptospires were found in 8/10 dogs, with PCR confirming bacteria as L. interrogans or L. kirschneri. Of these 10 dogs studied, 9 had previously been vaccinated against Leptospirosis, highlighting the potential for infection by serovars not present in vaccines.5
Because this is a zoonotic disease, it is prudent to understand the prevalence and incidence of Leptospirosis in wild populations of animals, in order to more effectively evaluate the domestic-wild animal interface as a potential risk factor for infection. A recent study investigated 98 animals (domestic and wild) found in areas adjacent to the Barcelona Metropolitan Area in Spain. While no statistically significant differences were observed between groups comprised of wild carnivores versus domestic carnivores, or between wild carnivores versus free-roaming cats, there was an overall prevalence of approximately 8.5%. This result corroborates previously published results in other studies, where prevalence varied based on infection host, geography, and proximity to water.6
Reports of Leptospirosis in cats has been infrequently published, though the prevalence of antileptospiral antibodies varies from 4% to 33.3%, geography dependent. With the consumption of infected rodent prey being implicated in transmission, cats spending time outdoors are at an increased risk of infection. Additionally, cats can be asymptomatic carriers, and continue to shed Leptospira in their urine, contaminating the environment and potentially increasing risk to human populations. The most frequently isolated serovars in infected cats in the United States include Australis, Autumnalis, Grippotyphosa, and Pomona. Interestingly, the serovars most often reported in clinical cases of acute leptospirosis include Autumnalis, Australis, Icterohaemorrhagiae, Grippotyphosa, Pomona, and Sejroe. The incomplete overlap between commonly detected versus clinical disease illustrates our incomplete understanding of this bacterium, and which serovars may cause only incidental infections. Cats are not currently vaccinated against Leptospiral infection, but an effective vaccine would be multivalent to cover many serovars.8
References:
1. Barocchi MA, Ko AI, Reis MG, McDonald KL, Riley LW. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but non intracellular pathogen. Infect Immun. 2002; 70: 6926?6932.
2. Chand N, Sanyal AJ. Sepsis-induced cholestasis. Hepatol. 2007; 45(1): 230-241.
3. Cianciolo RE, Mohr FC. Urinary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. 6th Vol. 2. St. Louis, MO:Elsevier Ltd; 2016:437?438.
4. Levett PN. Leptospirosis. Clin Micro Rev. 2001; 14(2): 296?326.
5. McCallum KE, Canstantino-Casas F, Cullen JM, et al. Hepatic leptospiral infections in dogs without obvious renal involvement. J Vet Intern Med. 2019;33:141-150.
6. Millan J, Velarde R, Chirife AD, Leon-Vizcaino L. Carriage of pathogenic Leptospira in carnivores at the wild/domestic interface. Polish Journal of Veterinary Sciences. 2019;22(4):781-784.
7. Miyahara S, Saito M, Kanemaru T, Villanueva SYAM, Gloriani NG, Yoshida S. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil's disease Int J Exp Path. 2014; 95:271?281.
8. Murillo A, Goris M, Ahmed A, Cuenca R, Pastor J. Leptospirosis in cats: Current literature review to guide diagnosis and management. Journal of Feline Medicine and Surgery. 2020;22:216-228.
9. Rissi DR, Brown CA. Diagnostic features in 10 naturally occurring cases of acute fatal canine leptospirosis. J Vet Diagn Invest. 2014; 26(6): 799?804.
10. Schuller S, Francey T, Hartmann K, Hugonnard M, Kohn B, Nally JE, Sykes J. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract. 2015; 56: 159?179.
11. Taylor C. Canine leptospirosis in the UK and Ireland. Vet Irel J. 2019; 9(5): 263-268.
12. World Health Organization. Wkly Epidemiol Rec. 2011; 86: 45?52.