Signalment:
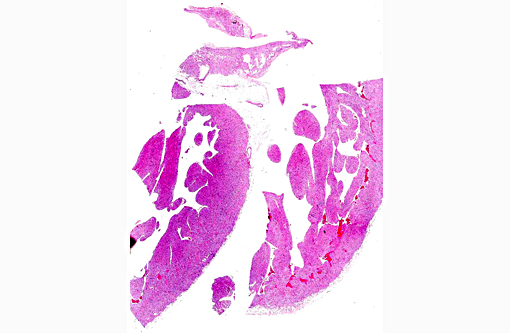
Gross Description:
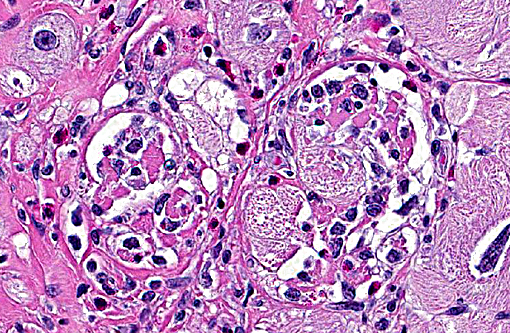
Histopathologic Description:
The right ventricle (not submitted) has minimal aggregates of mononuclear cells in the myocardial interstitium. No histological findings were present in the right auricle.
Other findings include passive congestion of the liver, mild hepatic capsular fibrosis, with subcapsular atrophy and focal hemorrhages. Desmin immune histochemistry demonstrated altered distribution in enlarged cardiomyocytes of the left ventricle, with focal decreased labeling of intercalated discs and Z bands and pale granular cytoplasmic staining, especially in areas of myocardial disarray.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
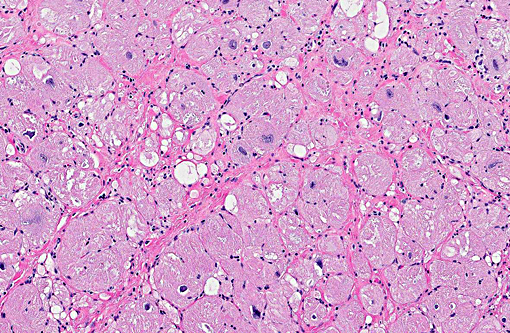
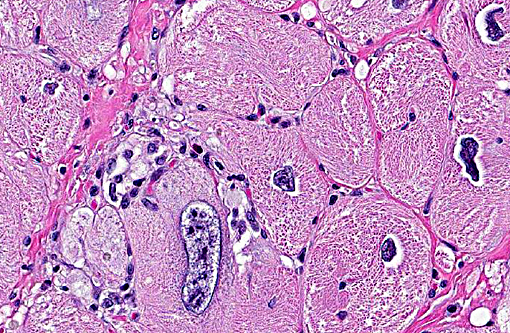
Hypertrophic cardiomyopathy can affect all chambers or be restricted to the left ventricle. Common histologic features include myocardial disarray, myocardial hypertrophy and vesicular nuclei, as well as diffuse interstitial fibrosis.(8,11) In general, domestic species have dilated cardiomyopathy and the dilated phase of hypertrophic cardiomyopathy have similar histologic features, which do not indicate etiology or the underlying mechanism of disease process. Histopathology by itself is not a definitive method of differentiation between these two diagnoses. In humans, hypertrophic cardiomyopathy is characterized by similar histological changes as described above in domestic animals. Myocardial disarray is considered a cardinal diagnostic feature of hypertrophic cardiomyopathy in humans when it occupies 20% of one or more tissues blocks.(4) The dilated phase of hypertrophic cardiomyopathy is usually associated with left ventricular myocardial fibrosis and remodeling, thinning of the ventricular wall and dilatation of the chamber, resembling macroscopic morphologic features of dilated cardiomyopathy.(5) Fibrosis is often associated with dysplastic changes in the media of small intramyocardial arteries.(2)
An important feature in human cases of hypertrophic cardiomyopathy is the immunohistochemical expression of desmin. Desmin is an intermediate filament found in muscle tissue, which forms a network around Z-bands, intercalated discs and myofibrils in cardiomyocytes.(3,4) In the case of this nonhuman primate, desmin immunostain displayed altered distribution in enlarged cardiomyocytes of the left ventricle, with granular cytoplasmic staining and decreased labeling of intercalated discs and Z bands, especially in the disarrayed myocardial fibers compared to unaffected areas in the same section. It was proposed that the lack of proper binding between defective myosin and other sarcomeric filaments causes defective sarcomere formation and myofibrillar disorganization, resulting in disarray and compensatory hypertrophy of cardiac myocytes in cats.(6) Based on this postulate, this author and others conclude that altered expression of desmin might serve as a possible immunohistochemical marker for hypertrophic cardiomyopathy.(3,4)
The electrocardiogram for this nonhuman primate revealed an inverted QRS complex on lead II, diagnostic of a Grade III heart block. Heart block can be the result of several disease states, including myocardial degeneration, necrosis and inflammation, when it occurs in close proximity of the conduction system (J&K). In humans, high grade ventricular block is a rare complication of hypertrophic cardiomyopathy although mild alterations of electrical conduction are commonly observed.(10) In non-human primates, cardiomyopathy has a fairly infrequent occurrence and there is a lack of literature regarding common ECG findings during the clinical course of disease. Interestingly, in both human and animals, histological changes of myocardial fibrosis and cardiomyocyte disruption have been reported secondary to stress cardiomyopathy, myocardial infarction, hypertension, cardiac hypertrophy and heart failure. Particularly in stress-induced cardiomyopathy (also known as Takotsubo cardiomyopathy), the literature includes descriptions of contraction band necrosis, leukocyte infiltrates and edema. In this condition, left ventricular dysfunction and wall abnormalities are described in the apical and midventricular myocardium, but the basal myocardium is spared.(7)
In cynomolgus monkeys, the most common spontaneous finding in the heart is focal mononuclear infiltrates in the interstitium or in perivascular regions, often from the subendocardium, with or without minimal myocardial degeneration and necrosis. This histologic change is not associated with any clinical condition and is considered incidental. Other less common spontaneous findings in macaques include mineralization of myocardium and/or blood vessels, endocarditis, pericarditis, eosinophilic infiltrates and fibrosis.(1)
Spontaneous cardiomyopathy was recently described in cynomolgus monkeys.(12) In this case series, there were no clinical or gross abnormalities. Findings were mainly histological, with extensive areas of myocardial disarray, interstitial fibrosis, vacuolation of perimyseal connective tissue, and disrupted myocytes containing vacuolated sarcoplasm and enlarged nuclei (karyomegaly). Despite the appearance of altered cardiomyocytes, this condition was not associated with active cardiomyocyte damage. In the present case, occasional infiltrates of mononuclear cells occurred in the right ventricle and were considered separate from the cardiomyopathy. In the left ventricle, inflammatory infiltrates (containing lymphocytes, macrophages and eosinophils) were the result of myocardial degeneration and necrosis with chronic remodeling, myocardial disarray and fibrosis. We believe that the case presented here is a completely separate entity that shares some of the histological features described by Zabka et al (2009)(12), but has a different clinical outcome and pathophysiology.
The main findings in this case are: a) electrocardiographic evidence of grade III heart block; b) the presence of a dilated and thin left ventricular free wall; c) myocardial disarray in histopathology, and d) decreased and granular sarcoplasmic desmin expression demonstrated by immunohistochemistry. Altogether, these findings support the diagnosis of dilated phase of hypertrophic cardiomypathy in this cynomolgus macaque.
JPC Diagnosis:
Conference Comment:
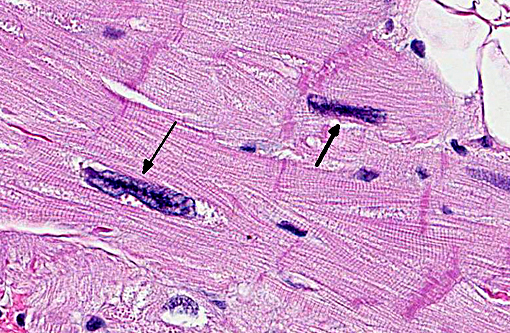
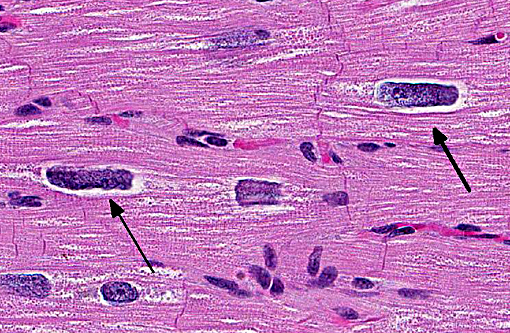
Approximately 60% of the myocardium is affected in the section. The most obvious change is the markedly cytomegalic and karyomegalic cardiac myocytes, varying in size from 10-100 times the normal macaque cardiomyocyte, with evidence of both hypertrophy and degeneration. A Massons trichrome stain highlights the severe and diffuse (rather than subendocardial) myocardial fibrosis, and the moderator discussed the fibrosis as being a separate distinctive process. In the moderators opinion, transverse sections of myocardium are best to evaluate for non-parallel orientation of cardiomyocytes (myofiber disarray); in this case he would grade the degree of disorganization as mild to moderate. The moderator led a discussion comparing hypertrophic cardiomyopathy (HCM) with cardiac hypertrophy, and in this case many participants were not able to reach a definitive diagnosis of HCM based on the histologic sections and information provided, although it was certainly considered with the differential diagnosis.
The cytologic appearance of the myocyte in this case is consistent with both HCM and myocardial hypertrophy but other differentiating histologic features were not identified. While myofiber disarray is the most significant diagnostic criteria for HCM, it is a relative rather than absolute change, and minor disarray can be evident with other cardiac diseases and even in normal hearts. Additionally, because there is usually asymmetric hypertrophy of the interventricular septum in HCM, concurrent evaluation of the IVS (not submitted) would aid in diagnosis. Fibrointimal dysplasia of small and medium mural coronary arteries is often a feature of HCM (particularly in the IVS), but was not evident in the examined section. HCM is generally considered to be a
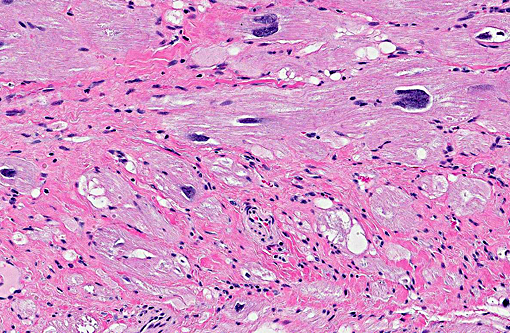
makes diagnosis more challenging. Contraction band necrosis was described by participants in the atrial myocardium, but was not a prominent feature, and is distinguished from hypercontraction artifact.
A subtle feature pointed out by the moderator is small tags of fibrous tissue extending from the pericardium, which provides evidence of pericardial effusion. Pericardial effusion and passive congestion in the liver are both features of right sided heart failure, and in this case the right ventricle (not submitted) was described by the contributor as having minimal changes. It is postulated that the marked degree of left ventricular hypertrophy likely compressed the right ventricular lumen, impairing function and resulting in pericardial effusion and passive congestion.
increases in both pressure and volume. Volume overload may also result in dilation of the ventricle lumen. Both mechanical stimuli and trophic stimuli can initiate pathologic hypertrophy, and result in changes such as increased protein synthesis and myocyte hypertrophy, which result from changes in gene expression. However, myocardial changes that occur in pathologic hypertrophy, including impaired contractility and relaxation, eventually limit the benefit of the compensatory hypertrophic response. Once hypertrophy is no longer able to compensate for the increased workload, degenerative changes occur in the myocardium secondary to factors such as poor vascular supply. Interstitial fibrosis may also result, leading to deterioration of function and eventual failure. Concentric hypertrophy typically results from increases in afterload and eccentric hypertrophy (dilated chamber) results from increases in preload.(9) Myofiber disarray is generally not a prominent feature of cardiac hypertrophy.
References:
1. Chamanza R, Parry NMA, Rogerson P, Nicol JR, Bradley AE. Spontaneous lesions of the cardiovascular system in purpose-bred laboratory nun-human primates. Toxicol Pathol. 2006; 34:357-363.
2. Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy. Pathology and pathogenesis. Histopathology. 1995; 26:493-500.
3. Francalanci P, Gallo P, Bernucci P, Silver MD, dAmati G. The pattern of desmin filaments in myocardial disarray. Human Pathol. 1995; 26:262-266.
4. Gallo P, dAmati G. Cardiomyopathies. In: Silver MD, Gotlieb AI and Schoen FJ, eds. Cardiovascular pathology. 3rd ed. pp. 285-325. Philadelphia, PA: Churchill Livingstone; 2001:285-325.
5. Hamada T, Kubo T, Kitaoka H, Hirota T, et al. Clinical features of the dilated phase of hypertrophic cardiomyopathy in comparison with those of dilated cardiomyopathy. Clin Cardiol. 2010; 33:E24-E28.
6. Hayman R, Une Y, Nomura Y. Desmin as possible immunohistochemical finding for feline hypertrophic cardiomyopathy. J Vet Med Sci. 2000; 62:343-346.
7. Lyon AR, Rees PSC, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008; 5:22-29.
8. Maxie MG, Robinson WF. Cardiovascular system. In: Maxie MG, ed.Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 3. 5th ed. Philadelphia, PA: Elsevier; 2007: 44-50.
9. Robinson WF, Robinson NA. Cardiovascular System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 3. 6th ed. St. Louis, MO: Elsevier; 2015:7-10, 45-47.
10. Tamura M, Harada K, Ito T, Enoki M, Takada G. Abrupt aggravation of atrioventricular block and syncope in hypertrophic cardiomyopathy. Arch Dis Child. 1995; 73:536-537.
11. Van Vleet JF, Ferrans VJ. Myocardial diseases of animals. Am J Pathol. 1986; 124:98-178.
12. Zabka TS, Irwin M, Albassam MA. Spontaneous cardiomyopathy in Cynomolgus monkeys (Macaca fascicularis). Toxicol Pathol. 2009; 37:814-818.