Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 22
15 April, 2020
CASE II: Case 2 (DVD) (JPC 4136179).
Signalment: 2.9 year old male rhesus macaque (Macaca mulatta)
History: The animal was assigned to a study investigating the effects of co-stimulatory blockade on delaying rejection of renal porcine xenotransplants. The animal presented with acute severe dyspnea one-month post-transplantation.
Gross Pathology: Red-tinged foam and liquid was present surrounding the nares and muzzle. Similar foam was present within the larynx and trachea. There was approximately 5mL of straw-colored fluid within the pleural cavity with few floating fibrin aggregates. The lungs were wet, heavy, failed to collapse, and were mottled red-pink.
Laboratory results:
Hematologic and serum biochemical analyses demonstrated a leukocytosis (11x103) with mild neutrophilia, lymphopenia, monocytosis, mild anemia, mild hypoproteinemia with low-normal albumin, mild azotemia, hyperphosphatemia, hyperglycemia, and hyperkalemia.
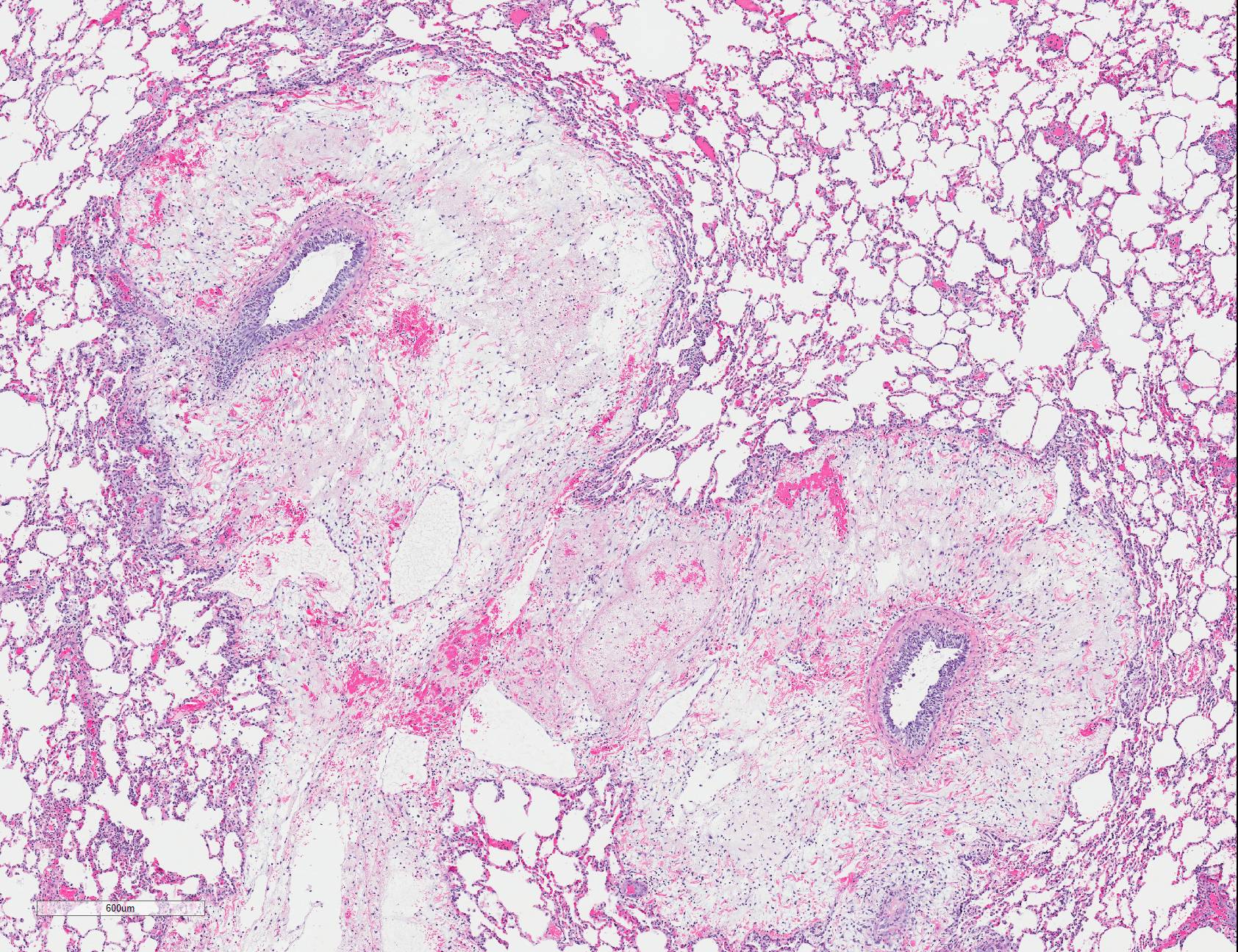
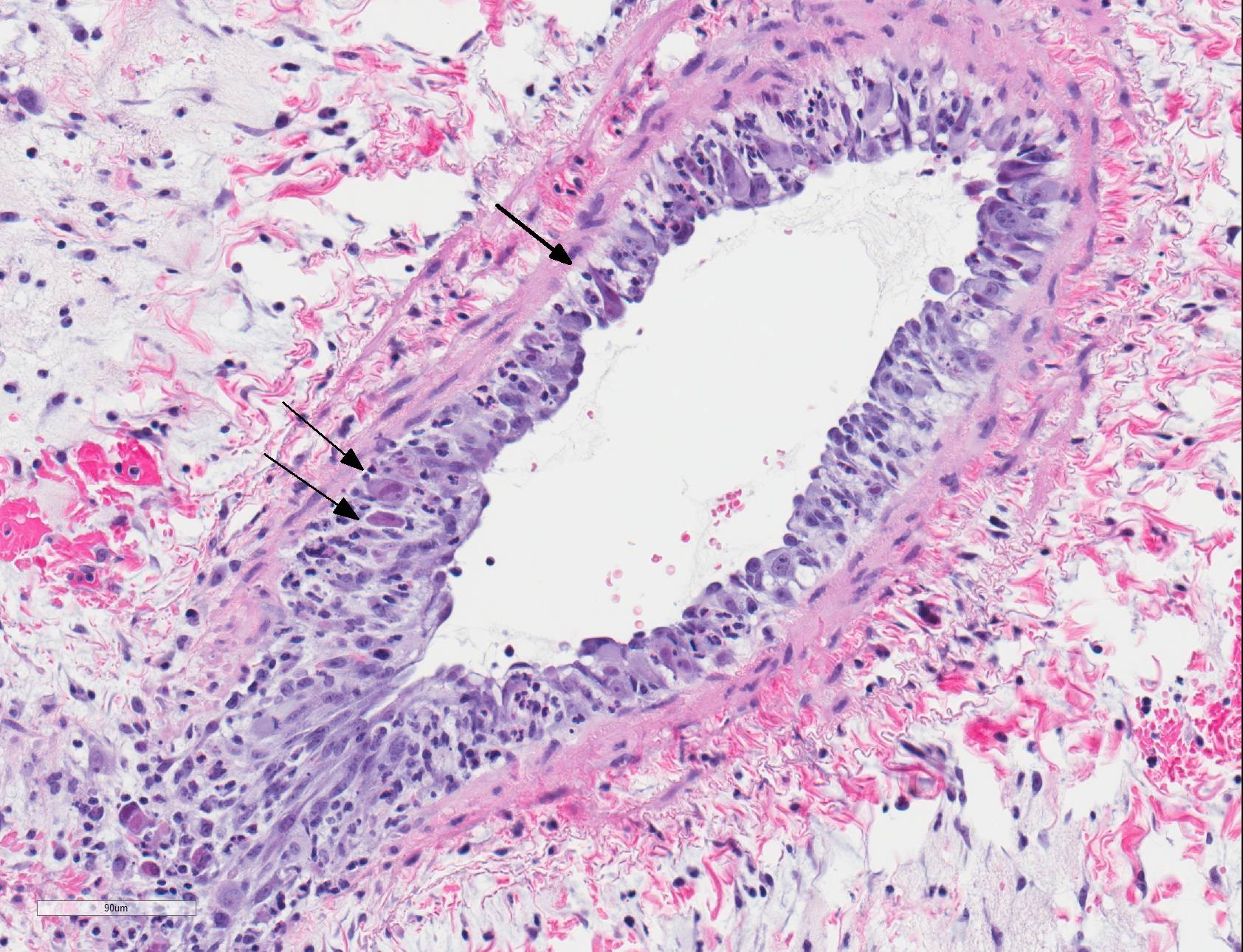
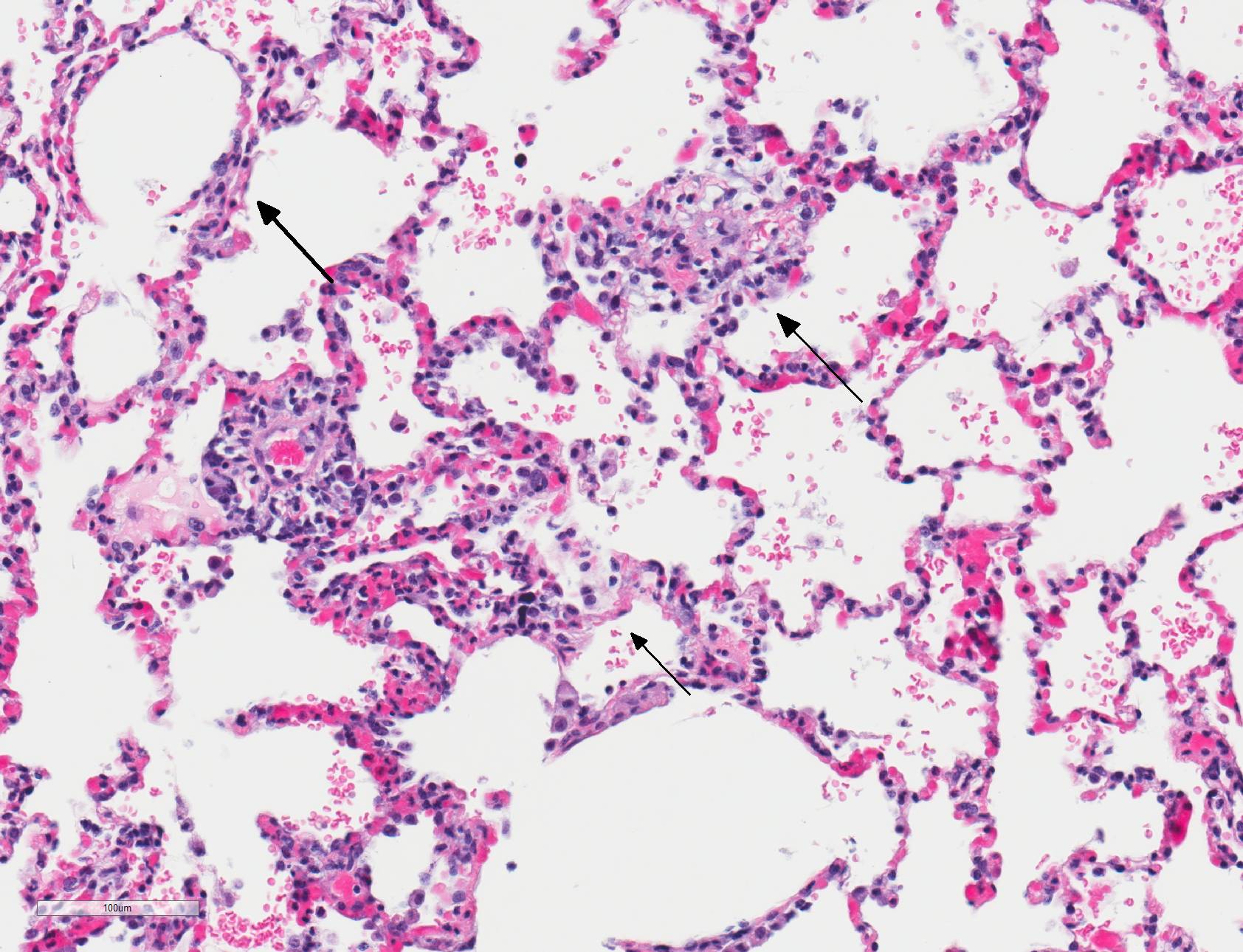
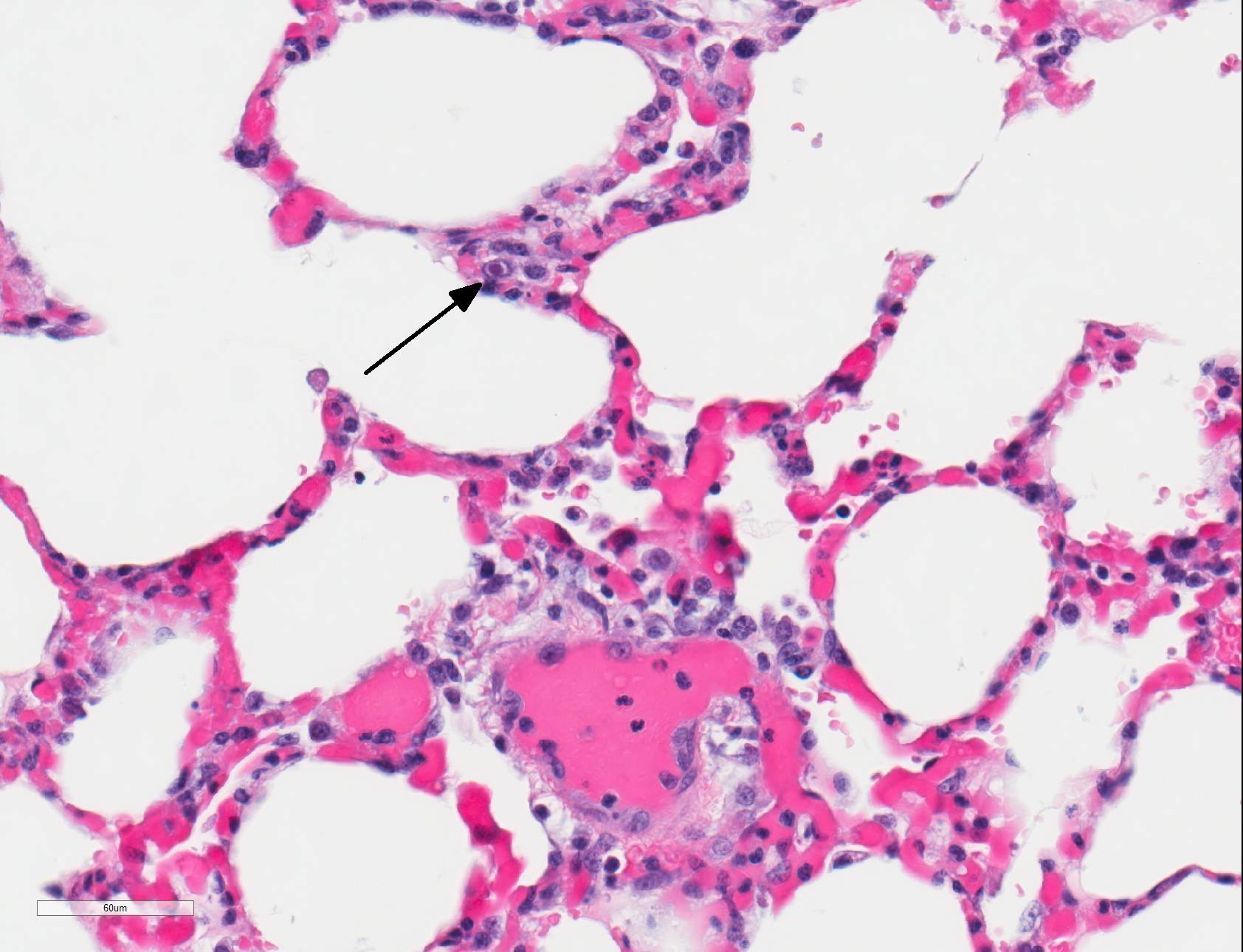
Microscopic Description: Lungs: There are numerous small and medium caliber blood vessels that are lined by endothelial cells with markedly enlarged nuclei and cytoplasm (cytomegaly) and occasional Cowdry type-A eosinophilic intranuclear inclusion bodies. Affected vessels are frequently infiltrated by neutrophils, lymphocytes, and histiocytes within the tunica media and intima (vasculitis). Multiple vessels are surround by abundant clear space (perivascular edema) and lesser amount of free erythrocytes (hemorrhage). Few larger vessels have abundant proliferation of the tunica intima with infiltration by neutrophils There are scattered coalescing regions where alveolar spaces contain free erythrocytes, fibrin, neutrophils, necrotic debris, and increased numbers of alveolar macrophages that frequently contain abundant eosinophilic cytoplasm (alveolar edema). There are multiple patchy regions where alveolar septae are expanded by similar mixed inflammatory cells. There are rare intranuclear inclusion bodies present within histiocytes and type-1 pneumocytes.
Contributor?s Morphologic Diagnosis:
Lungs: Severe, multifocal widespread, neutrophilic and lymphocytic small and medium artery vasculitis with endothelial cytomegaly, Cowdry type-A intranclear inclusions (cytomegalovirus) and perivascular edema; Mild to moderate, multifocal to coalescing, neutrophilic and lymphocytic interstitial pneumonia with alveolar edema and hemorrhage.
Contributor?s Comment: Rhesus cytomegalovirus (macacine herpesvirus 3), is a double-stranded DNA virus in the family betaherpesvirus that is commonly identified in non-specific pathogen free colonies of rhesus macaques, with 50% of infants seropositive by 6 months of age and almost 100% by 1 year old.22 Transmission is believed to be horizontal, as vertical transmission is exceedingly rare, which mimics the human virus4. Infections are usually asymptomatic with the virus remaining latent until an immunosuppressive event occurs, allowing for recrudescence.
Specifically, T-cells play an important role in controlling CMV replication,16 which are also the target of many immunosuppressive therapies in organ transplantation and in SIV and type-D retroviral infections.1 Cytomegaloviral infection/recrudescence has been reported as a complication leading to death or euthanasia in multiple nonhuman primate species undergoing solid organ or stem cell transplantation, including rhesus13,15 and cynomolgus12 macaques and baboons.2
Although it is difficult to assess the complication rates in nonhuman primate transplant studies due to the relatively small numbers of animals reported in the literature, the rate of post-transplantation infections is thought to be 14%.10 These infections can be classified as early (<1 month), intermediate (1-6 months), or late (>6 months) infections. Generally, early infection etiologies most frequently include bacteria or Candida17, while intermediate infections are typified by the viral infections such as cytomegalovirus, Epstein-Barr virus, varicella zoster virus, herpes B virus, human herpesvirus-6, and simian immunodeficiency virus.10 It is probably more accurate to consider these as re-emergent latent pre-transplantation viral infections rather than de novo post-operative infections.
In humans, CMV is most commonly associated with HIV and transplant-associated immunosuppression.8,19,20 CMV-related diseases in these cases include chorioretinitis, gastrointestinal diseases (colitis, esophagitis, gastritis, hepatitis), pneumonia, encephalitis and myelitis, and adrenal adenitis. Lesions associated with cytomegaloviral infection in nonhuman primates result from disseminated infections, which are almost always linked to immunosuppression. Diseases resulting from disseminated infection include: necrotizing enteritis, encephalitis, lymphadenitis, and/or pneumonitis/interstitial pneumonia.1 Typically, alveolar septa are lined by hypertrophied type 2 pneumocytes and contain cytomegaly with large intranuclear Cowdry type-A inclusion bodies within the septa and lining. Alveolar spaces contain fibrin, alveolar macrophages, and neutrophils. Similar lesions can also be found in spleen, liver, kidney, and testis. The similarities between humans and nonhuman primates regarding pathogenesis and gross and histologic lesions makes nonhuman primates an ideal model for studying CMV in immunosuppressed patients.
Within the present case, only pulmonary lesions (and resultant hydrothorax) were observed, with classical cytomegaloviral inclusions observed predominately within vascular endothelial cells, and alveolar septa and macrophages much less frequently observed. Endotheliitis in the lungs and other organs from cytomegalovirus has also been described in immunosuppressed humans.9,21 Vasculitis is a less common pathologic finding than alveolitis, but can cause perivascular and alveolar edema and hemorrhage, as well as pleural effusion.11,18 In rhesus macaques, CMV has also been reported to cause hypophysitis5 and facial neuritis3 with co-infection with SIV, and mesenchymoproliferative enteropathy with co-infection with SIV and simian polyomavirus.14
Contributing Institution:
Division of Pathology, Yerkes National Primate Research Center, Emory University
http://www.yerkes.emory.edu/research/divisions/pathology/index.html
JPC Diagnosis: Lung: Pneumonia, interstitial, necrotizing and neutrophilic, multifocal to coalescing, moderate, with necrotizing vasculitis and endothelial and pneumocyte karyomegalic viral inclusions.
JPC
Comment: The
contributor has provided an excellent and concise review of cytomegalovirus,
and their importance in both primate and transplant research.
Present in most if not all mammalian species, cytomegaloviruses (CMV) are
slow-growing viruses that result in a life-long latent infection, with
recrudescence and clinical disease only in times of severe immunosuppression.
In order to do this, cytomegaloviruses have developed unique methods of
preventing the host from clearing the infection, which are only now beginning
to come to light.
As shown in the mouse model (murine cytomegalovirus is a well-researched agent due to its similarity to human CMV), toll-like receptors and cellular stress responses will activate intrinsic methods of cell death following CMV infection. In order to combat this, CMV has developed ways to counteract a number of key factors which inhibit cellular death, i.e., "death inhibitors".6
One
method is a viral protein which inhibits mitochondrial outer membrane
permeabilization, a key step in apoptosis, by producing vMIA (viral
mitochondria-localized inhibitor of apoptosis), which inhibits the activator Bak
and sequesters Bax at the mitochondrial membrane.6
CMV also produces a viral protein, UL38, which results in accumulation of the
activating transcription factor 4 (ATF4) and suppression of JNK activity. ATF4
helps to resolve cell stress by inducing the production of proteins that
facilitate protein folding within the ER, and the inactivation of JNK inhibits
activation of Bim and Bcl-2, further stabilizing the mitochondrial membrane.6
CMP infection also modifies so-called "death receptors" at the cell surface, such as the TNF-related apoptosis-inducing ligand (TRAIL). An open reading frame on M166, a CMV viral-encoded protein inhibits the expression of this receptor at the cell surface. Other viral proteins inhibit Fas-induced cell death by binding to pro-caspase 8 and inhibiting its activation.6 Several proteins also inhibit activators of null killer cells at the level of the NKG2D cell receptor (CD226).7
References:
1. Abee CR, Mansfield K, Tardiff S, Morris T: Nonhuman Primates in Biomedical Research, Second ed., pp. 19-20. Elsevier, Oxford, 2012
2. Asano M, Gundry SR, Izutani H, Cannarella SN, Fagoaga O, Bailey LL. Baboons undergoing orthotopic concordant cardiac xenotransplantation surviving more than 300 days: effect of immunosuppressive regimen. J Thorac Cardiovasc Surg. 2003;125: 60-69; discussion 69-70.
3. Assaf BT, Knight HL, Miller AD. Rhesus Cytomegalovirus (Macacine Herpesvirus 3)?Associated Facial Neuritis in Simian Immunodeficiency Virus?Infected Rhesus Macaques (Macaca mulatta). Veterinary Pathology. 2014;52: 217-223.
4. Barry PA, Lockridge KM, Salamat S, et al. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J. 2006;47: 49-64.
5. Berg MR, Owston MA, Gauduin M-C, Assaf BT, Lewis AD, Dick EJ. Cytomegaloviral hypophysitis in a simian immunodeficiency virus-infected rhesus macaque (Macacca mulatta). Journal of Medical Primatology. 2017;46: 364-367.
6. Brune W, Andoniou CE. Die another day: inhibition of cell death pathways by cytomegalovirus. Viruses 2017; 9(9):doi 10.3390/v9090249.
7. De Pelsmaeker S, Romero N, Vitale M, Favoreel HW. Herpes virus evasion of natural killer cells. J Virol 2018; 92(11): doi 10.1128/JVI.02105-17.
8. . Drew WL. Cytomegalovirus infection in patients with AIDS. Clin Infect Dis. 1992;14: 608-615.
9. . Golden MP HS, Wanke CA, Albrecht MA. Cytomegalovirus Vasculitis- Case Reports and Review of the Literature. Medicine. 1994;73: 246-255.
10.. Haustein S, Kolterman A, Sundblad J, Fechner J, Knechtle S. Nonhuman primate infections after organ transplantation. ILAR J. 2008;49: 209-219.
11. . Herry I CJ, Antoine M, Meharzi J, Michelson S, Parrot A, Rozenbaum W, Mayaud C. Cytomegalovirus-induced alveolar hemorrhage in patients with AIDS: a new clinical entity? Clin Infect Dis. 1996;22: 616-620.
12. Jonker M, Ringers J, Kuhn EM, t Hart B, Foulkes R. Treatment with anti-MHC-class-II antibody postpones kidney allograft rejection in primates but increases the risk of CMV activation. Am J Transplant. 2004;4: 1756-1761.
<13. Kean LS, Adams AB, Strobert E, et al. Induction of Chimerism in Rhesus Macaques through Stem Cell Transplant and Costimulation Blockade-Based Immunosuppression. American Journal of Transplantation. 2007;7: 320-335.
14. Macri SC, Knight HL, Miller AD. Mesenchymoproliferative Enteropathy Associated With Dual Simian Polyomavirus and Rhesus Cytomegalovirus Infection in a Simian Immunodeficiency Virus?Infected Rhesus Macaque (Macaca mulatta). Veterinary Pathology. 2012;50: 715-721.
15. Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74: 933-940.
16. Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005;79: 381-386.
17. Rubin R, Schaffner A, Speich R. Introduction to the Immunocompromised Host Society Consensus Conference on Epidemiology, Prevention, Diagnosis, and Management of Infections in Solid-Organ Transplant Patients. Clinical Infectious Diseases. 2001;33: S1-4.
18. Salomon N GT, Perlman DC, Laya L, Eber C, Mildvan D. Clinical features and outcome of HIV-related cytomegalovirus pneumonia. AIDS. 1997;11: 319-324.
17. Schulman LL RD, Austin JH, Rose EA. Cytomegalovirus pneumonitis after cardiac transplantation. Arch Intern Med. 1991;151: 1118-1124.
19. Sia IG, Patel R. New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipients. Clin Microbiol Rev. 2000;13: 83-121, table of contents.
20. Smith FB, Arias JH, Elmquist TH, Mazzara JT. Microvascular Cytomegalovirus Endothelialitis of the Lung. Chest. 1998;114: 337-340.
21. Vogel P, Weigler BJ, Kerr H, Hendrickx AG, Barry PA. Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab Anim Sci. 1994;44: 25-30.