CASE 1: 19764-13 (4048438-00)
Signalment:
3.75 year old neutered male Labrador retriever mix (Canis lupus familiaris)
History:
A gait change was first noted by the owner at 2.5 years of age. By January 2013, overt pelvic limb ataxia and nail wear was observed, and the patient was beginning to develop fecal and urinary incontinence. Second examination in May 2013 reveals worsening ataxia and reduced pelvic limb reflexes. By September 2013 the dog was non-ambulatory, paraparetic and was euthanized. At the time of euthanasia, the bladder had to be manually expressed and vocalizations were hoarse.
MRI revealed T2 intensification in the T7 vertebral body, but none in the spinal cord. CBC-chemistry values normal when tests were done in January. The dog was genotypically normal when tested for SOD1 mutation found in canine degenerative myelopathy in February 2013.
Gross Pathology:
No lesions were noticed during preparation of tissues from microscopic examination.
Laboratory results:
None.
Microscopic description:
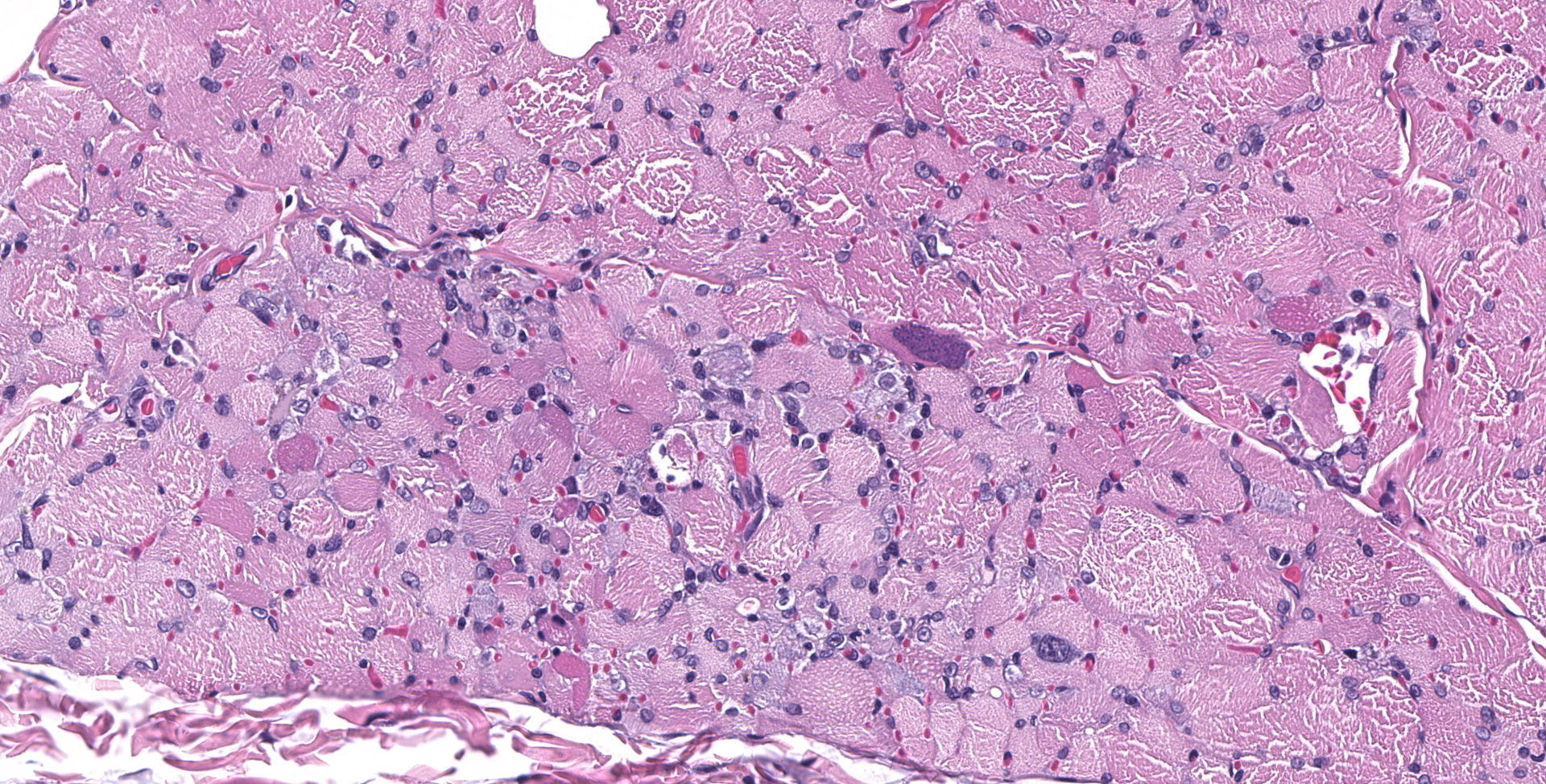
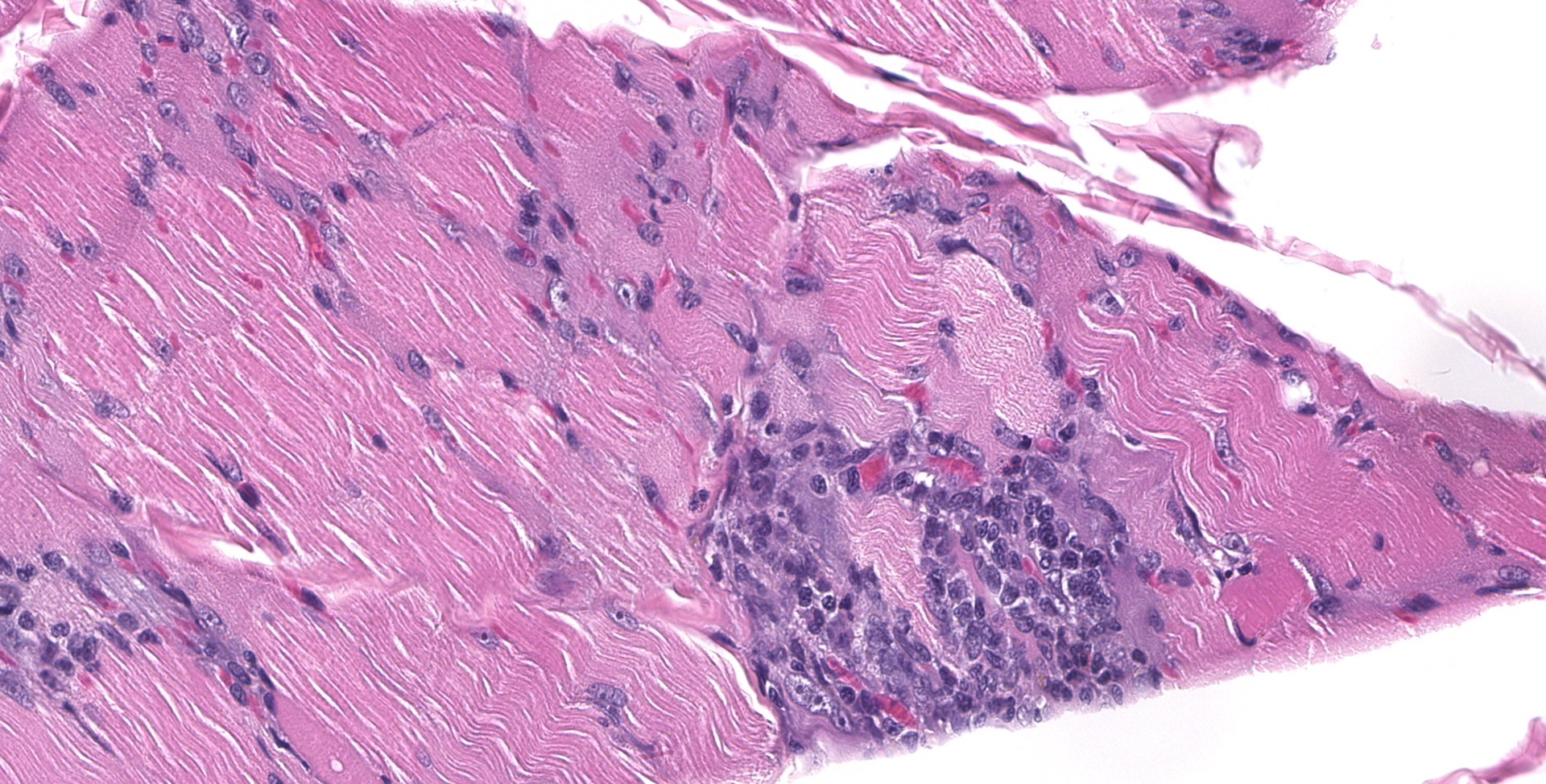
BICEPS FEMORIS MUSCLE: Scattered foci of lymphocytes and macrophages occur in the interstitium of the muscle. Degenerating and regenerating muscle fibers are associated with this inflammation. In addition, degeneration and necrosis of individual muscle fibers occur where inflammation is absent. These contain more frequent clusters of neutrophils. Protozoal trophozoites and cysts are present in the lesions. Some muscle bundles contain small angular fibers as well, and some of the nerves between muscle bundles appear to contain a reduced number of axons.
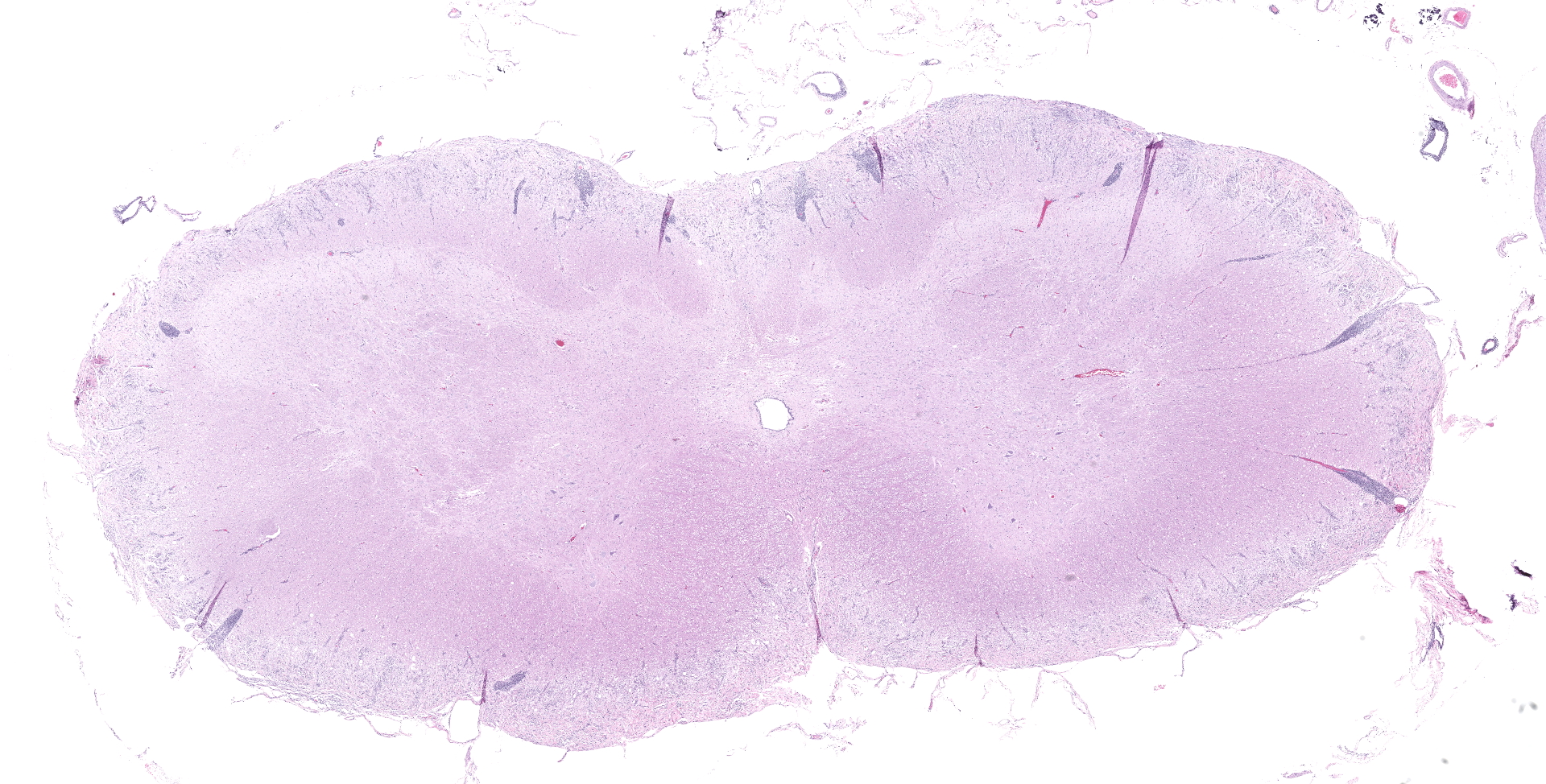
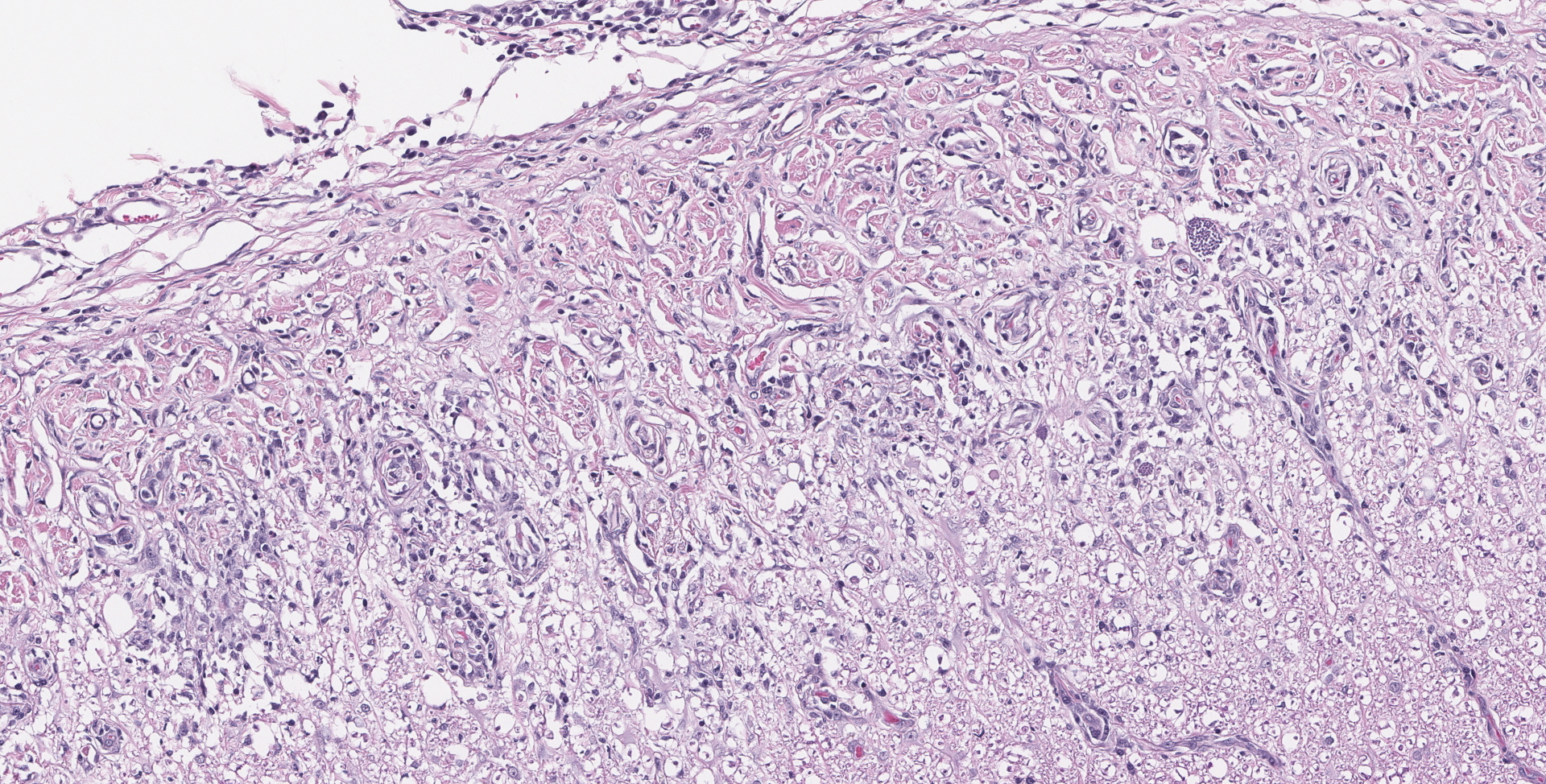
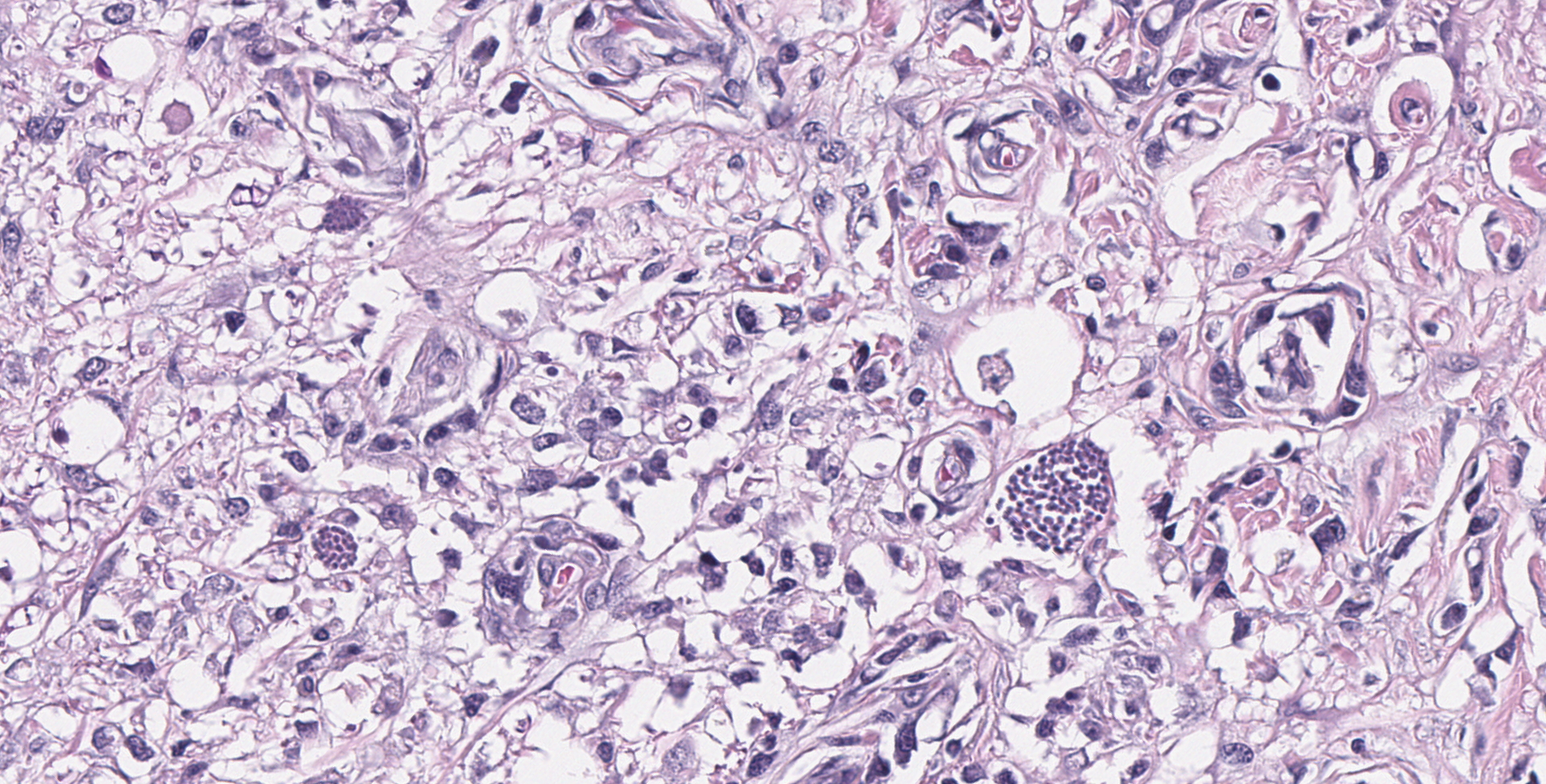
SPINAL CORD: Sections of spinal cord are characterized by vacuolation of the subpial white matter, with extensive astrogliosis. Penetrating coronal vessels have plump endothelium and are surround by variably thick lymphohistiocytic cuffs. In the tissue, there are small clusters of plasma cells and rare nests of neutrophils. The leptomeningeal infiltrate consists largely of plasma cells. The affected tissue contains coccidian protozoal cysts with bradyzoites and less common individual tachyzoites. Enlarged astrocyte nuclei are evident in the affected areas. Similar lesions were present in the brainstem, cerebellum, pons and medulla with mild cerebral lesions. As well as occurring near the pial surface, lesions were common near the ependymal lining of the brain ventricles.
Contributor's morphologic diagnosis:
Widespread, surface-oriented lymphohistiocytic encephalomyelitis with plasmacytic meningitis and numerous coccidian protozoa
Widespread multifocal lymphohistiocytic myositis, with localized neurogenic atrophy and frequent coccidian protozoa
Contributor's comment:
This dog developed slowly progressive ataxia, beginning as an adult. At the time of death, over a year later active proliferation of Neospora was apparent in muscle and throughout the neuraxis. This animal had no history of immuno-suppressive therapy. It is not clear whether this case represents reactivation of a silent infection of primary progressive infection of an adult animal.
Neospora caninum is an apicomplexan protozoan parasite causing acute and recrudescent infections, primarily of dogs and cattle. Viable organisms can be isolated from cattle, water buffalo, sheep, dogs, horses, bison and miscellaneous wild ruminants and canids.6 Vertical transmission is considered variable in dogs and may not persist for repeat transmission during pregnancy.6 It is known that dogs can harbor encysted parasites in several organs, as detected by PCR.10 However, vertical trans-mission from the dam to puppies, sometimes with disease, has also been documented.8,12 But, experimentally, not all dogs given 10,000 oocysts shed organisms or seroconverted.2 Horizontal fecal transmission has not been proved, but dogs can shed organisms after being fed infected tissue. Dogs can also be infected with Toxoplasma gondii, Sarcocystis neurona5 and Sarcocystis spp17 which can be confused with Neospora if immunohistochemical or PCR confirmation is not performed.
It is known that various isolates of Neospora caninum can vary in virulence.7 Using proteomics, Regidor-Cerrillo et al identified tachyzoite proteins associated with higher virulence15 that seemed to accentuate the gliding motility of the organism and increase oxidative stress. Some Neospora isolates killed about 10% of fetuses in a murine model of canine infection, while others were less pathogenic.4
Whether adult dogs can undergo reactivation of infection leading to clinical disease is a matter of speculation. Neospora caninum has been known to cause disease in adult dogs undergoing immunosuppressive therapy,9,14,16 and the contributor has observed an additional case that resulted in localized severe myositis with numerous organisms.
Detecting infection can be problematic: it has been estimated that up to 32% of Algerian pound dogs were infected as tested by PCR, and there was poor correlation between PCR results and seropositive test results among individuals.10
In cattle, abortions occur after oocysts are ingested during pregnancy or with reactivation of previous infection.6 There is an estimated 40-60% rate of vertical transmission, although not all fetal infections produce abortion. Most abortions occur at 5-6 gestational months, and less common CNS infection has been detected in calves less than 2 months of age. No predisposing immunosuppressive event is generally detected that might provoke reactivation.
Much has been done to try to distinguish reactivation from primary infection from chronic infection serologically. Cattle with antibody to both tachyzoite and bradyzoite antigen are suspected of reactivation. Anti-GRA7 (dense granule protein) antibody is found in acute infection or reactivation, while SAG4 (bradyzoite specific) antibody rise indicates chronic infection.2 Cell-mediated immunity is potentially needed to control infection and is predominantly Th1.13 Treatment with IFN-? or TNF-? was shown to reduce tachyzoites.
Contributing Institution:
Veterinary Medical Diagnostic Lab
University of Missouri
VMDL.missouri.edu
JPC diagnosis:
1. Spinal cord: Meningomyelitis, necroti-zing and lymphohistiocytic, diffuse, moderate, with numerous apicomplexan cysts, Labrador retriever, canine.
2. Skeletal muscle: Myositis, lymphohistiocytic, multifocal, moderate, with intracellular apicomplexan cysts.
3. Skeletal muscle: Atrophy, segmental, marked.
JPC comment:
There was discussion in conference that there may have been some slide variation, with some participants receiving sections of brainstem as well as spinal cord. The morphologic diagnosis will differ slightly, but the pathologic process remains the same.
The contributor provides a concise summary and explanation of this case. Neospora spp have three distinct infectious stages: tachyzoites, tissue cysts, and oocysts, with tachyzoites and tissue cysts found in both intermediate and definitive hosts. As stated by the contributor, N. caninum does not cause significant disease in adult cattle but causes abortion in beef and dairy cattle. Some common lesions in fetal calves include lymphocytic, plasmacytic, sometimes histiocytic hepatitis, pancarditis or myocarditis, myositis, and placentitis. The most common CNS lesion in bovine fetuses is multifocal discrete foci of necrosis, primarily in the brain, and the spinal cord to lesser extent. While capable of causing disease in dogs of all ages, the most common CNS lesions are necrotizing granulomatous, lymphoplasmacytic, and sometimes eosinophilic meningoencephalomyelitis, with tachyzoites often visible in lesions.3
The innate immune system is important in recognition of Neospora caninum. Both TLR2 and TLR3 play a role in initial recognition and induce production of IL-12 and IFN-?. However, recent research has centered on nucleotide oligomerization domain (NOD)-like receptors (NLR). A specific group of NLRs sense multiplying pathogen associated molecular patterns (PAMP) or damage associated molecular patterns (DAMP) and initiate the formation of the inflammasome. The NLRP3 inflammasome sense extracellular ATP, nigericin, and uric acid crystals, as well as many bacteria, viruses, fungi, and parasites. Research using murine peritoneal macrophages showed that to activate the NLRP3 inflammasome, two signals are required. The first is usually provided by NF-kb which results in increased transcription of NLRP3 and pro-IL-1b and pro-IL-18. The second signal is downstream and initiated by the first step, and then causes inflammasome complex formation, resulting in caspase-1 proteolytically cleaving pro-IL-1b and pro-IL-18 to their active forms. Concurrently, caspase-1 also triggers pyroptosis by cleaving gasdermin-D (GSDMD), resulting in GSDMD-formed pores in the plasma membrane. In murine macrophages exposed to N. caninum, gene expression was significantly increased for NLRP3 (and NLRC4 and NLRC5 to a lesser extent). In Nlrp3-/- macrophages, significantly lower amounts of IL-1b and IL-18 were produced, resulting in a less robust immune response to the pathogen.18,19
Neospora caninum has been detected in various avians as well, such as the chicken, sparrow, magpie, and buzzard. A recent finding in Iran was that a number of hooded crow (Corvus cornix) brains were PCR positive for either Neospora caninum, Toxoplasma gondii, but no coinfections were detected. The crows most likely become infected after ingesting oocysts from infected carrion, but their role in the lifecycle remain to be elucidated more fully.1
A potentially emerging disease in China, Neospora caninum has been detected in pigs and reported for the first time. Serum samples collected from January 2017 until December 2018 were tested and approximately 1.9% of all samples were positive for antibodies via a competitive-inhibition enzyme-linked immuno- sorbent assay (cELISA). Concurrently, PCR testing of brain samples was positive for N. caninum genes Nc-5 and the ITS-1 region, finding five and eight in 600 samples harbored the parasite, respectively. The testing shows that pigs can serve as an intermediate host for N. caninum, but the epidemiological significance is not yet clear.11
References:
1. Abdoli A, Arbabi M, Pirestani M, et al. Molecular assessment of Neospora caninum and Toxoplasma gondii in hooded crows (Corvus cornix) in Tehran, Iran. Comparative Immunology, Microbiology and Infectious Diseases. 2018; https://doi.org/10.1016/ j.cimid.2018.06.008.
2. Aguado-Martinez A, Alvaez-Garcia G, Fernández-Garcia A, et al. Usefulness of fNcGRA7- and rNcSAG-based ELISA tests from distinguishing prim-infection, recrudescence, and chronic bovine neosporosis. Vet Parasitol. 2008;157:182-195.
3. Cantile C, Youssef S. Nervous System. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, 6th Ed, Vol 1. St. Louis, MO: Elsevier. 2016:387-388.
4. Dellarupe A, Regidor-Cerillo J, Jiménez-Ruiz E, et al. Clinical outcome and vertical transmission variability among canine neospora caninum isolates in a pregnant mouse mode of infections. Parasitol. 2014;141:356-366.
5. Dubey JP, Black SS, Verma SK, et al. Sarcocystic neurona schizonts-associated encephalitis, chorioretinitis and myositis in a two-month-old dog simulating toxoplasmosis, and presence of mature sarcocysts in muscle. Vet Parasitol. 2014; 202:194-200.
6. Dubey JP, Schares G. Neosporosis in animals ? the last five years. Vet Parasitol. 2011;180:90-108.
7. Dubey JP, Steekumar C, Knickman E, et al. Biologic, morphologic and molecular characterization of Neospora caninum isolates from littermate dogs. Intern J Parasitol. 2004;334:1157-1167.
8. Dubey JP, Vianna MC, Kwok OC, et al. Neosporosis in beagle dogs: clinical signs, diagnosis, treatment, isolation and genetic characterization of Neospora caninum. Vet Parasitol. 2007;149:158-166.
9. Fry DR, McSpooran JD, Ellis JT et al: Protozoal hepatitis associated with immunosupressive therapy in a dog. J Vet Intern Med. 2009;23:366-368.
10. Ghalmi F, China B, Kaidi R, et al.Comparison of different serological methods to detect antibodies specific to Neospora caninum in bovine and canine sera. J Vet Diagn Invest. 2014;26:136-140.
11. Gui B, Lv Q, Ge M, Li R, Zhu X, Liu G. First report of Neospora caninum infection in pigs in China. Transbound Emerg Dis. 2019;00:1-4.
12. Heckeroth AR, Tenter AM. Immunoanalysis of three litters born to a Doberman bitch infected with Neospora caninum. Parasitol Res. 2007;100:837-846.
13. Jesus EE, Pinheiro AM, Santos AB, et al. Effects of IFN-?, THF-?, IL-10 and TGF-? on Neospora caninum infection in rat glial cells. Exp Parasitol. 2013;133:269-274.
14. La Perle KM, del Piero F, Carr RF, et al. Cutaneous neosporosis in two adult dogs on chronic immunosuppressive therapy. J Vet Diagn Invest. 2001;13:252-255.
15. Regidor-Cerrito J, Álvarez-Garcia G, Pastor-Fernández I, et al. Proteome expression changes among virulent and attenuated Neospora caninum isolates. J Proteomics. 2012;75:2306-22318.
16. Saev V, Martlé V, Van Ham L, et al. Neuritis of the cauda equina in a dog. J Small Anim Pract. 2010;51:549-552.
17. Sykes JE, Dubey JP, Lindsay LL, et al. Severe myositis associated with Sarcocystis spp, infection in 2 dogs. J Vet Intern Med. 2011;25:1277-1283.
18. Wang X, Gong P, Zhang X, et al. NLRP3 Inflammasome Participates in Host Response to Neospora caninum infection. Frontiers in Immunology. 2018;9:1791.
19. Wang X, Gong P, Zhang X, et al. NLRP3 inflammasome activation in murine macrophages caused by Neospora caninum infection. Parasites and Vectors. 2017;10:266.