Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 23
15 April, 2020
Paul C. Stromberg, DVM, DACVP
Professor Emeritus
Department of Veterinary Biosciences
The Ohio State University College of Veterinary Medicine
Columbus, OH
CASE III: 61290 (JPC 4117380).
Signalment: 7.5 year old, female Malayan Snail-eating turtle, Malayemys subtrijuga
History: This captive-hatched animal was found dead with no history of clinical signs.
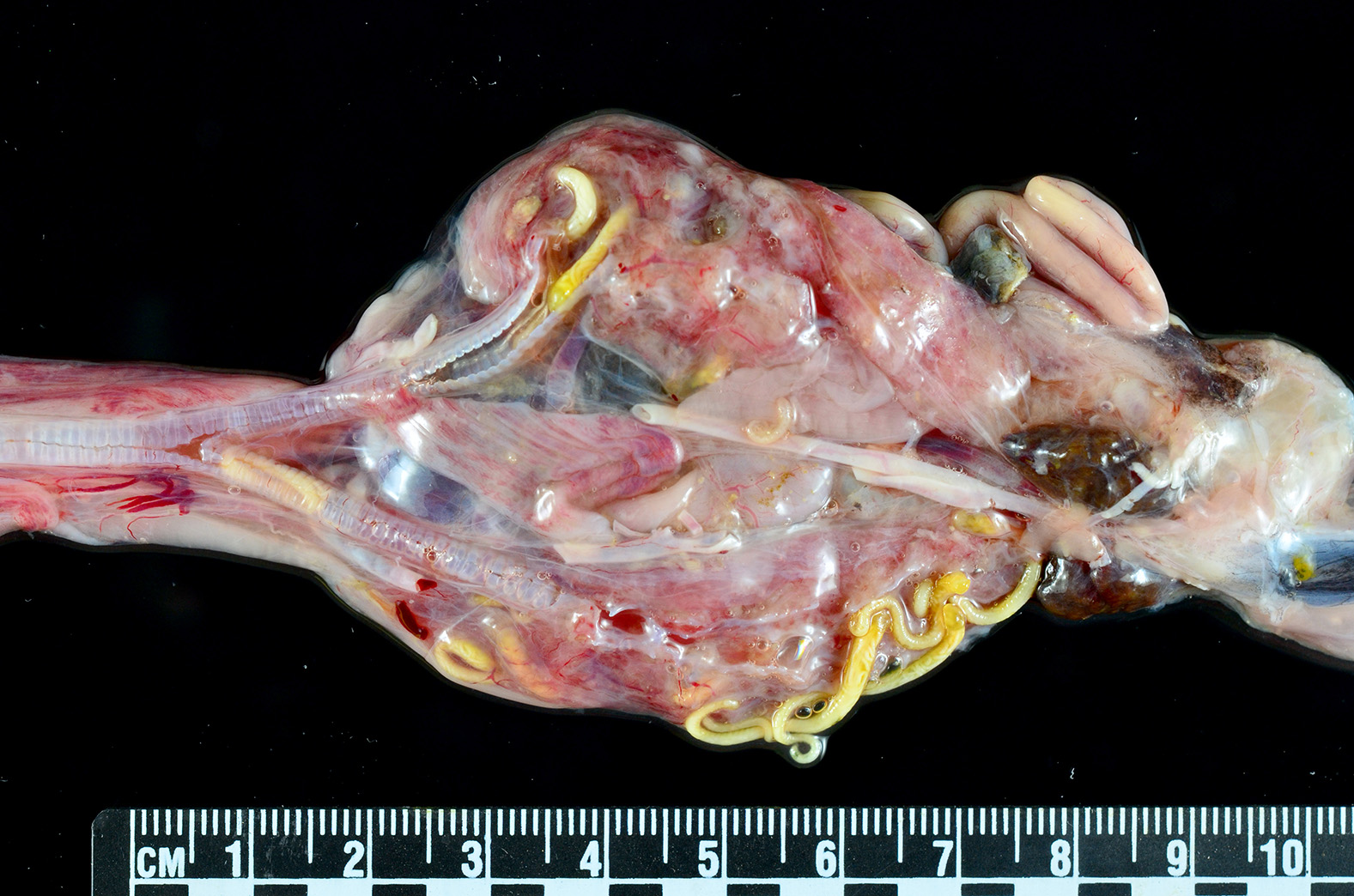
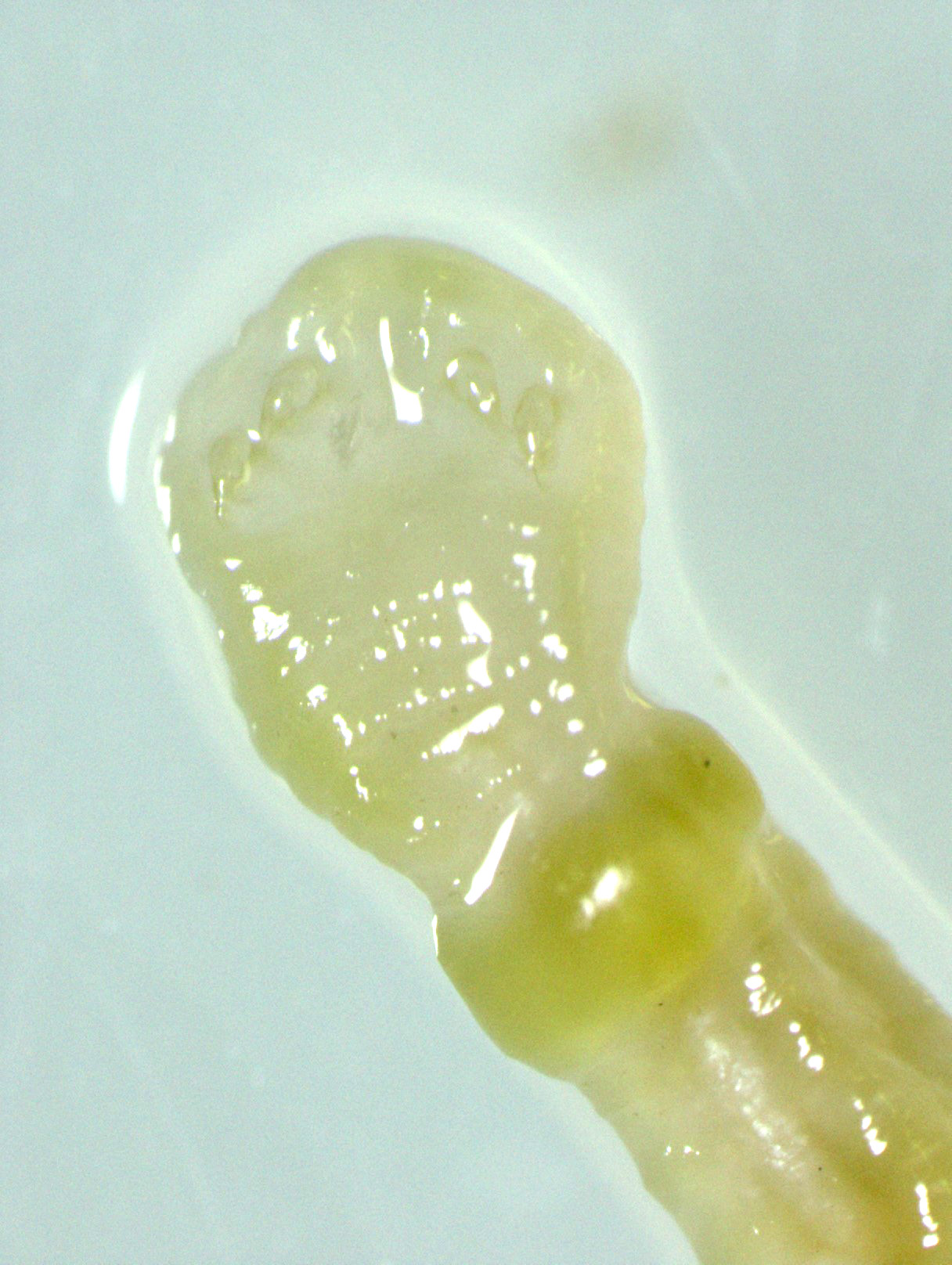
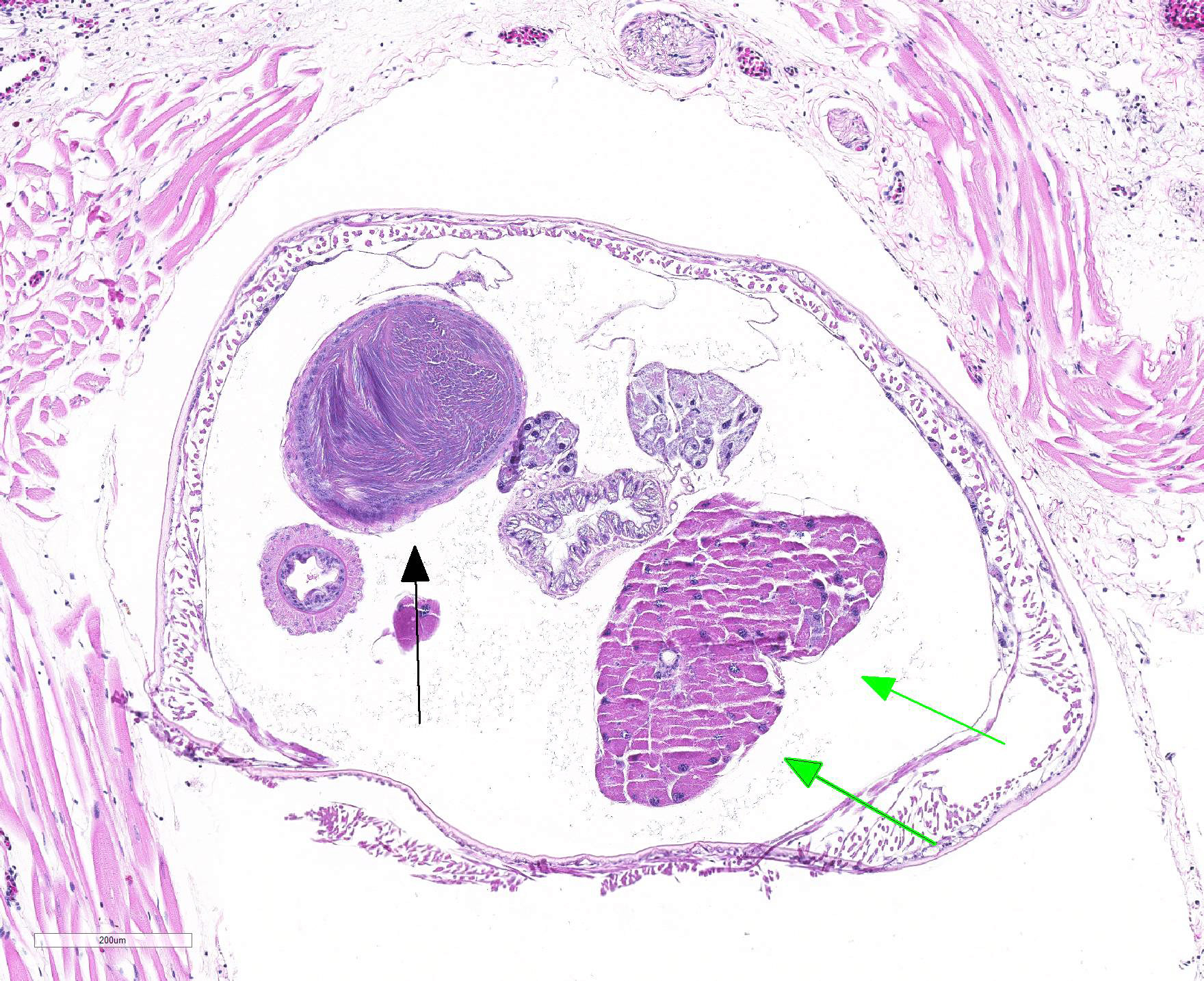
Gross Pathology: An approximately 2.0 cm long by 0.3 cm diameter off-white to yellow, round parasite (identified as a pentastome) was within the caudal oral cavity near the base of the tongue. Approximately 5-10 similar parasites were throughout the parenchyma of each lung, and a single parasite was folded within the lumen of the right bronchus (Figure 1). Under a dissecting scope, the parasites had a slightly expanded and flattened anterior end with four hooks on the flattened surface (Figure 2). There was very subtle banding or pseudosegmentation of the parasite body, and most of the body cavity was filled with a thick, highly convoluted white to off-white tubular structure (presumed uterus, Figure 3). The turtle?s lungs were diffusely pink-red and wet, yet floated when placed in 10% neutral buffered formalin. Two smaller grey-tan, coiled, round pentastome nymphs measuring approximately 1.0 cm long by 0.1 cm diameter were in the adventitia of the mid esophagus, adjacent to the medial aspect of the left lung. No adipose stores were present, and there was some watery coelomic fluid.
Laboratory results: Parasite Identification: Whole parasites were submitted to the Zoological Medicine and Wildlife Disease Laboratory at the University of Florida for speciation using a pentastome PCR assay, which resulted in a sequence that identified the parasites as Sebekia mississippiensis.
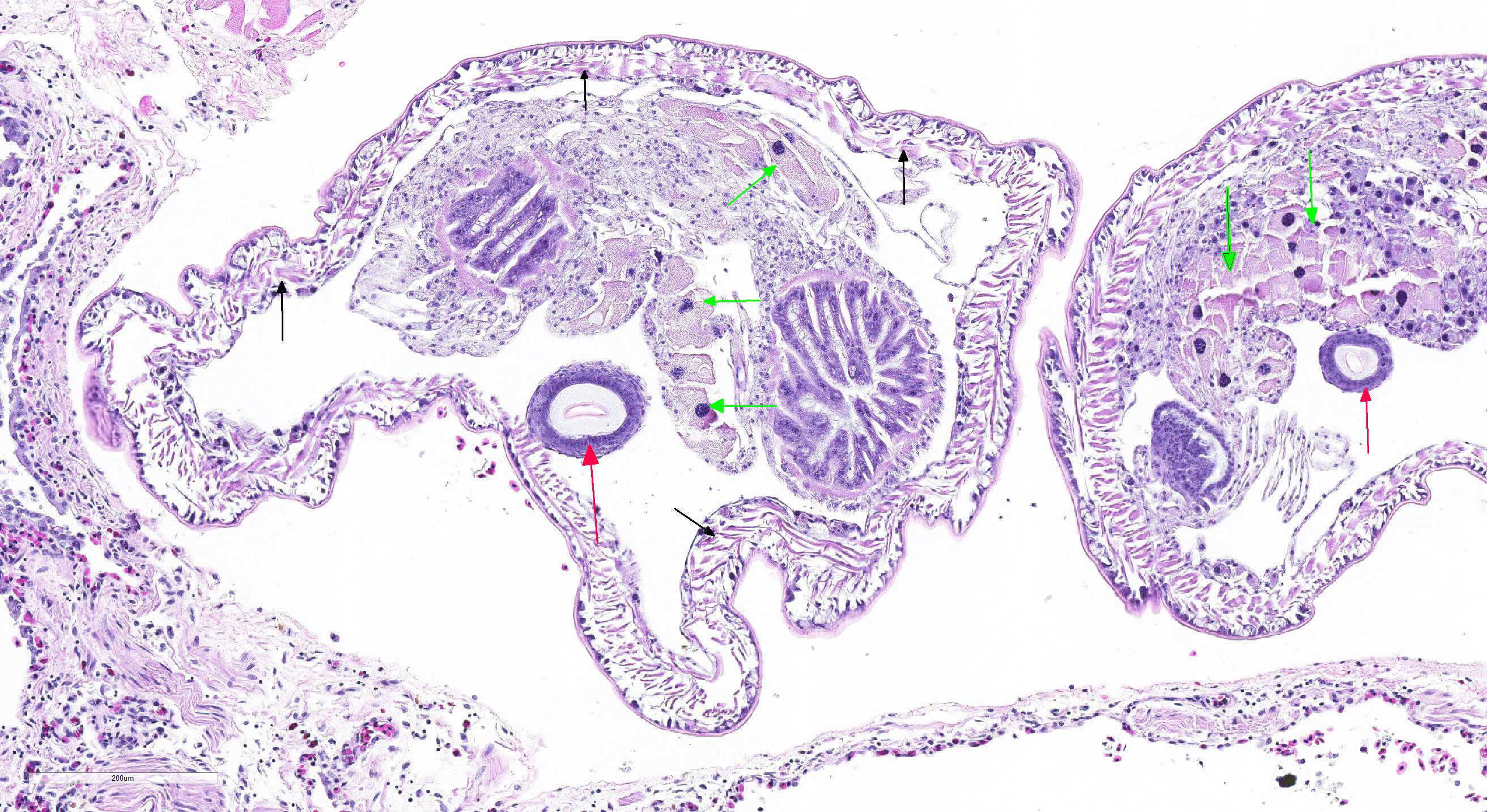
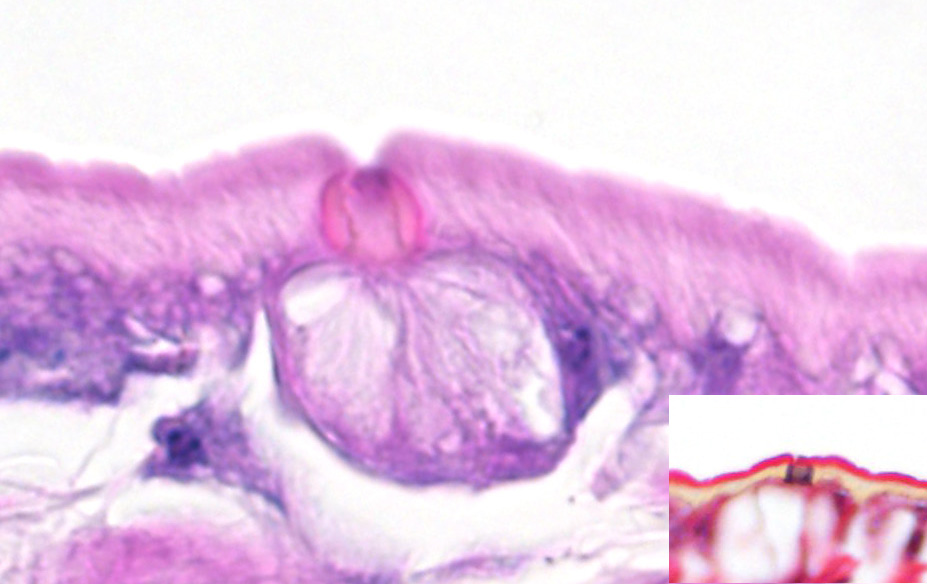
Microscopic Description: Lung (sections from 3 blocks): There is some variability between slides. The interstitium of faveolar septa is diffusely expanded by edema and variable numbers of inflammatory cells with occasional foci of hemorrhage. The inflammatory infiltrate consists of predominately granulocytes, lymphocytes and plasma cells with fewer histiocytes and melanomacrophages. A few 1-2 mm diameter metazoan parasites expand and compress faveoli, fill a bronchial lumen, or are embedded in pleural connective tissue, depending on the section. These parasites have features consistent with pentastomes, including a thin, undulating to pseudosegmented eosinophilic cuticle which contains occasional openings lined by refractile, eosinophilic material (sclerotized pores) (Figure 4). Underlying the cuticle, striated skeletal muscle fascicles with subcuticular glands make up the body wall, which encloses a body cavity containing a multicellular digestive tract lined by eosinophilic glands and a reproductive tract (Figure 5). In sections of mature parasites, the uterus is filled with developing eggs. Chitinous hooks are present in some sections. Also present in faveolar lumina are variable amounts of sloughed respiratory epithelium mixed with granulocytes, macrophages, red blood cells, debris and bacteria. The faveolar epithelium is frequently absent, hypertrophied or hyperplastic. There are 1-2 discrete heterophilic granulomas within the interstitium in some sections. These occasionally contain folded, hyalinized, eosinophilic membranous material (presumed pentastome cuticle).
Contributor?s Morphologic Diagnosis:
Lungs: Moderate, diffuse, granulocytic and lymphoplasmacytic interstitial pneumonia with epithelial erosion and hyperplasia and intralesional adult and nymph pentastomes
Contributor?s Comment: Pentastomiasis is a disease caused by a group of blood-sucking endoparasites with a world-wide distribution.5 Variably considered a separate phylum or a subclass of phylum Arthropoda, Pentastomida is an interesting group of obligate parasites most closely related to branchiurans, or fish lice, a type of crustacean arthropod.5,7 The distribution of definitive hosts and fossil records show pentastomids first appeared when reptiles flourished during the Mesozoic era.7,9 Approximately 90% of pentastomes use reptiles as definitive hosts, and the life cycle is indirect.7,9
Adult pentastomes typically reside in the lower respiratory tract and produce larvated ova that are swallowed and expelled from the definitive host in feces.5 Intermediate hosts of various pentastomids are mostly fish and mammals, which can include humans, with additional animals able to serve as paratenic hosts.7 Some definitive hosts can harbor nymph and adult stages.7,9 The intermediate host ingests ova from contaminated water, vegetation, or fecal matter. Once ingested, the first stage nymph emerges from the egg, penetrates the gastrointestinal tract, and migrates through viscera. Here the nymph encysts and molts multiple times to become an infective nymph. Once the intermediate host is ingested by the definitive host, nymphs excyst and migrate to the lungs where they mature, completing the lifecycle.5,7
Infections in reptile hosts vary in severity from incidental to fatal. Adult pentastomes in their natural host often incite only mild inflammation; however, clinical signs in captive reptiles can include lethargy, anorexia, dyspnea, pneumonia, and sudden death.1,4,5 The eggs can be detected in feces or lung wash samples.2,4 Clinical signs are due to tissue destruction or inflammation associated with parasite migration, from parasite molting which can elicit a hypersensitivity reaction, from respiratory tract obstruction, or from secondary bacterial infections.4,7,9 The turtle in this report had significant inflammation throughout the lungs, including a few heterophilic granulomas that appeared to be associated with molted cuticle, as well as obstruction of a primary bronchus by an adult worm.
The most defining characteristic of pentastomids in histologic tissue sections is the presence of sclerotic pores that open into the cuticle within the body wall. These pores produce cuticle during molts.2 Better visualization of the pores can be achieved by using a Movat pentachrome stain that stains the typically eosinophilic sclerotic pore black. Additional identifying features include two paired hooks surrounding the mouth, striated musculature, acidophilic glands encircling the intestinal tract, and pseudosegmentation.2
Malayan snail-eating turtles are carnivorous turtles with diverse diets including items such as fish, earthworms, aquatic insects, and, of course, snails. Its diet in captivity consisted of earthworms, processed turtle brittle and gel, and occasional snails. Fish were not reported to be component of its diet, although mosquito fish were sometimes present in the water system in which it was housed. The pentastomid species recovered from this turtle was Sebekia mississippiensis, for which fish (including mosquito fish) are intermediate hosts.1,6 Sebekia sp. are pentastomids of crocodilians, and Sebekia mississippiensis is specifically of the American alligator.1, 6 High mortality rates among hatchling crocodilians infected with Sebekia sp. has been previously reported, along with a single report of a dermal infection in a woman.1,6,7 Pentastomes are less frequently reported in turtles than other reptiles, but infections with Diesingia (family Sebekidae) have been seen.4,7,9 Both adults and nymphs were present in this turtle, which could indicate autoinfection or a continued source of reinfection. It could also act as both a definitive and intermediate host. A second snail-eating turtle in the collection was subsequently screened for pentastome infection and was negative. Screening of fish and proper freezing protocols prior to feeding can help reduce incidence of this parasite in susceptible captive animals.1,7
Contributing Institution:
Disease Investigations
Institute for Conservation Research
San Diego Zoo Global
PO Box 120551
San Diego, CA 92112
http://institute.sandiegozoo.org/disease-investigations
JPC Diagnosis: 1. Lung: Adult pentastomes, multiple, with mild lymphohistiocytic interstitial pneumonia.
2. Lung: Granuloma, heterophilic, focal.
JPC Comment: The contributor has presented an excellent review of pentastome infections in reptiles. To expand on their histology, pentastome have two pairs of hooks surrounding the mouth which led early researchers to believe that they had five heads (leading to the name ?pentastomes?).2 The presence and staining of sclerotized openings (unique to pentastomes) with Movat?s pentachrome is important as these structures are present in all life stages, and is maintained even in degenerate or calcifed specimens.2
Pentastomes utilize a wide range of intermediate hosts, and
occasionally end up in human intermediate hosts. Most human infections involve
the species Armillifer armillatus (whose definitive host is the
python) or A. grandis. Intermediate hosts are infected by eating water
and vegetation contaminated with eggs passed in the feces of respiratory
secretions of snakes.
Human infections are most common in regions of African where snake meat is
eaten, although some infections are likely acquired by ingesting water or
vegetation contaminated with the feces or respiratory secretions of snakes. Autopsy
studies have demonstrated a 22% infection rate in certain African countries,
and a 45% incidence in autopsy is some parts of Malaysia.8
Poorly cooked snake meat may be purchased at markets and eaten by inhabitants
of certain parts of Africa, and adult pentastomes (referred to locally as
"snake springs") are spit out.10 Chewing the pentastomes may result
in liberation of eggs. After ingestion, 4-legged primary larvae migrate
through the viscera, and following several molts, transformed in the legless
nymphs which are characteristically found on serosal membranes and rarely
within viscera.10 While most infections in these regions are the result
of A. armillifer, a significant number of cases of pentastomiasis may be
seen with A. grandis.8 While cases of A. armillifer
result in more traditional peritoneal encysted nymphs, A. grandis has a
predilection for ocular infections, with blindness as a common result.
References:
1. Adams L, Isaza R, Greiner E. Fatal Pentasomiasis in Captive African Dwarf crocodile Hatchlings (Osteolaemus tetraspis). Journal of Zoo and Wildlife Medicine. 2001;32(4): 500-502.
2. Gardiner, CH, Poynton, SL. An Atlas of Metazoan Parasites in Animal Tissues. Washington DC: Armed Forces Institute of Pathology; 1999 59-60.
3. Ioannou P, Vamvoukaki R. Armillifer infections in humans: a systematic review. Trop Me and Inf Disease 2019; 16;4(2). pii: E80. doi: 10.3390/tropicalmed4020080.
4. Jacobson ER. Infectious Disease and Pathology of Reptiles. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2007 590-592.
5. Meyers WM, Neafie RC. Pentastomiasis. In Meyers, WM, ed: Topics on the Pathology of Protozoan and Invasive Arthropod Disease. Ebook; 2011: 1-10.
6. Moreland, AF, Forrester, DJ,Delany MF. Sebekia mississippiensis (Pentastomida) from Juvenile American Alligators in North Central Florida. The Helminthological Society of Washington. 1989;56(1): 42-43.
7. Paré JA. An Overview of Pantastomiasis in Reptiles and Other Vertebrates. Journal of Exotic Pet Medicine. 2008;17(4): 285-294.
8. Potters I, Desaive C, Van Den Broucke S, Esbroeck MJ, Lynen L. Unexpected infection with Armillifer parasites. Emerg Inf Dis 2017; 23(12): 2116-2118.
9.
Riley
J. The Biology of Pentastomids. Advances in Parasitology. 1986;25: 45-128.
10.
Tappe D, Sulyok M, Riu T, Rozsa L, Bodo I, Schoen C, Muntau
B, Babocsay G, Hardi R. Co-infections in visceral pentastomiasis, Democratic
Republic of the Congo. Emerg Inf Dis 2016; 22(8):1333-1339.