Signalment:
Gross Description:
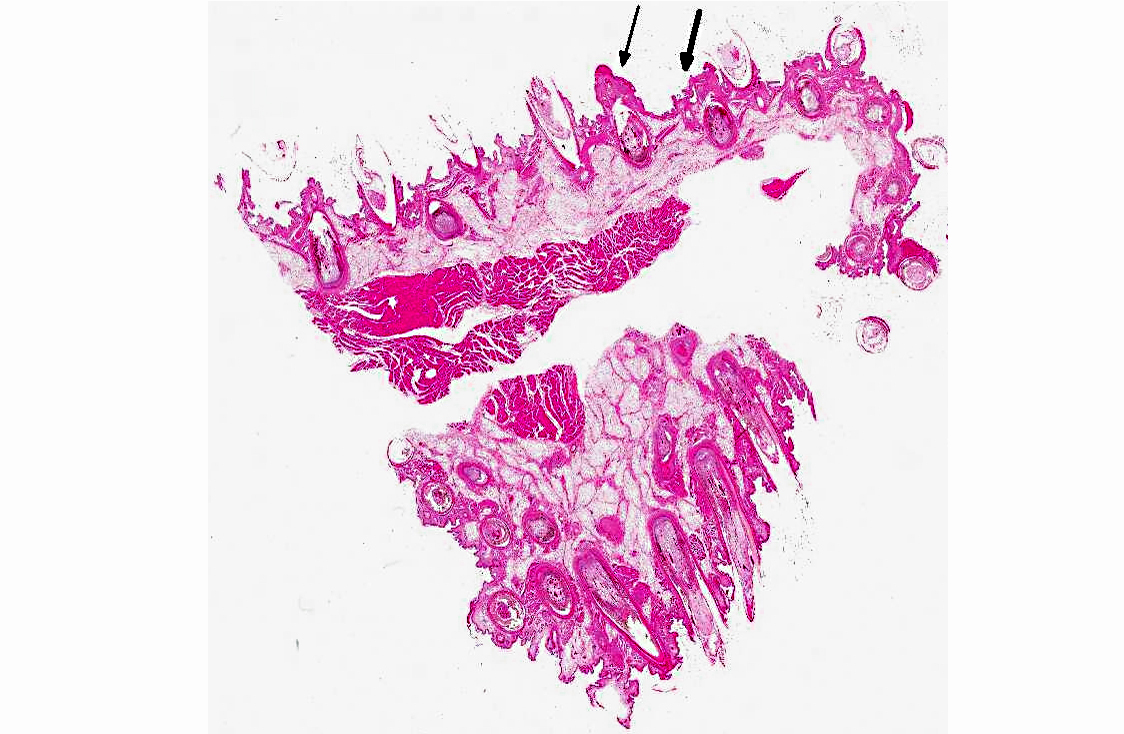
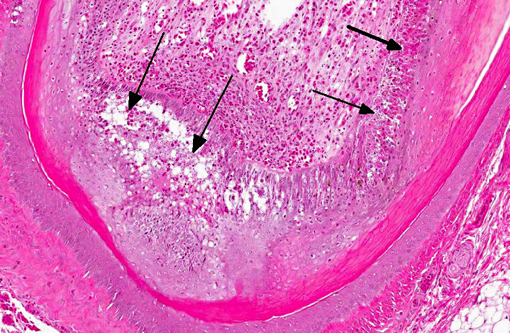
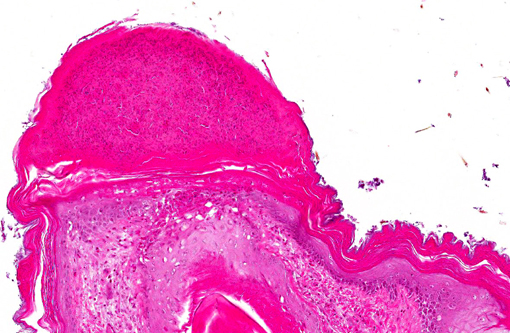
Histopathologic Description:
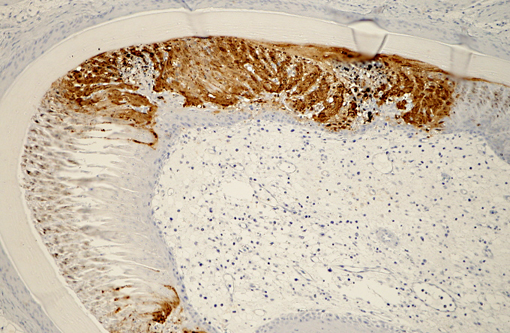
Immunohistochemical analysis to detect type A influenza virus revealed that influenza virus matrix antigens were present in the feather epidermal cells with/without necrotic changes. Few fibroblasts in the feather pulp were also positive for viral antigens. No relation was found between the virus antigen distribution and foci of lymphocytes in the dermis. Other major pathological findings in this case were non-suppurative encephalomyelitis, myocarditis, pancreatic necrosis, myositis, keratitis and focal epithelial necrosis of the beak, tongue and legs.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Histopathological findings in waterfowl infected with Asian H5N1 HPAI virus are frequently found in the central nervous system, heart and pancreas.(5) In addition, virus replication in the feather epidermis is one of the characteristic findings in waterfowl infected with H5N1 HPAI virus.(7) This microscopic feather lesion was reported in domestic ducks, geese and wild swans.(3,7,8) Even asymptomatic ducks had the feather lesions in the experimental infection.(7) Spherical virions were observed in the feather epidermis by electron microscopic examination.(7) These findings raise the possibility that H5N1 HPAI viruses may be released from feathers of infected waterfowl to the environment and that feathers could become potential sources of infection along with their feces and respiratory secretions.Â
JPC Diagnosis:
Conference Comment:
Highly pathogenic avian influenza (HPAI) is confined to subtypes H5 and H7, and is generally introduced into poultry flocks via wild birds (especially ducks).(4) Low-pathogenicity avian influenza (LPAI), replicates in the gastrointestinal tracts of waterfowl, who shed high concentrations of the virus in feces and have been implicated as an important viral reservoir.(3) LPAI infections in domestic birds are typically subclinical (with occasional mild respiratory signs or decreased egg production), however ,mutation to highly pathogenic avian influenza can occur, with devastating economic affects.(5) Resulting HPAI viruses can cause severe systemic disease in chickens, with necrosis and inflammation in the skin, viscera and brain. In general, the emergence of new and varied influenza viruses depends on genetic drift (point mutations) as well as genetic shift (genomic segment reassortment).(4) An important virulence determinant in avian influenza is the amino acid sequence at the hemagglutinin protein cleavage site. Low pathogenicity strains of virus have a single, basic amino acid (arginine) at the cleavage site; insertions, deletions or point mutations resulting in changes to this cleavage site can significantly alter pathogenicity.(4)
In poultry, following binding of hemagglutinin to host cell α2,3-glactose receptors and the subsequent induction of receptor-mediated endocytosis, avian influenza virus replicates in (and is shed from) both the respiratory and gastrointestinal tracts. Cell damage occurs secondary to direct virus replication, inflammatory mediators and/or vascular thrombosis and ischemia.(5) Depending on the host, HPAI can be epitheliotropic, endotheliotropic, neurotropic or pantropic.(5) The presence of gross findings depends upon the strain and virulence of the virus, and may include ruffled feathers; edema of the comb, wattles, periorbital areas and legs, subcutaneous hemorrhage; multifocal visceral and mucosal hemorrhage and necrosis; pulmonary edema and hemorrhage; pancreatic necrosis; and intestinal lymphoid necrosis.(5) Microscopic lesions are more frequent than gross lesions and consist primarily of necrosis and inflammation within multiple organs, especially the skin/feather follicles, pancreas, brain, heart, lungs, adrenal glands and primary/secondary lymphoid organs.(5) Central nervous system involvement can occur after direct viral spread from the nasal cavity to the brain via olfactory nerves, hematogenous spread, or infection of ependymal cells with subsequent ventriculitis/periventriculitis.(5) Death can be peracute, or it may follow multi-organ failure; extremely virulent strains of avian HPAI virus can cause up to 75% or even 100% mortality.(4,5) Similar morbidity and mortality has also been reported in turkeys, quail, guineafowl and pheasants.(5)
Early research demonstrated that HPAI viruses rarely produced fulminant disease in wild birds; however, since 2002 a new Eurasian-African lineage of H5N1 HPAI virus has been reported to cause clinical disease and death in ducks under both natural and experimental conditions.(3,5,6) As noted by the contributor, lesions in waterfowl infected with HPAI virus frequently occur in the brain, heart and pancreas, with characteristic virus replication in the feather epidermis,(7,8) as demonstrated in this case.
References:
2. Gilbert M, Chaitaweesub P, Parakamawongsa T, Premashthira S, Tiensin T, Kalpravidh W, Wagner H, et al. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg Infect Dis. 2006;12:227-234.
3. L+�-�ndt BZ, Nunez A, Banks J, Nili H, Johnson LK, Alexander DJ. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathol. 2008;37:619-627.
4. MacLachlan NJ, Dubovi EJ, eds. Fenners Veterinary Virology. 4th ed. London, UK: Elsevier; 2011:353-368.
5. Pantin-Jackwood MJ, Swayne DE. Pathogenesis and pathobiology of avian influenza virus infection in birds. Rev Sci Tech. 2009;28:113-136.
6. Sturm-Ramirez KM, Ellis T, Bousfield B, Bissett L, Dyrting K, Rehg JE, Poon L, et al. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J Virol. 2004;78:4892-4901.
7. Yamamoto Y, Nakamura K, Okamatsu M, Yamada M, Mase M. Avian influenza virus (H5N1) replication in feathers of domestic waterfowl. Emerg Infect Dis. 2008;14:149-151.
8. Yamamoto Y, Nakamura K, Yamada M, Ito T. Zoonotic risk for influenza A (H5N1) infection in wild swan feathers. J Vet Med Sci. 2009;71:1549-1551.