Signalment:
Gross Description:
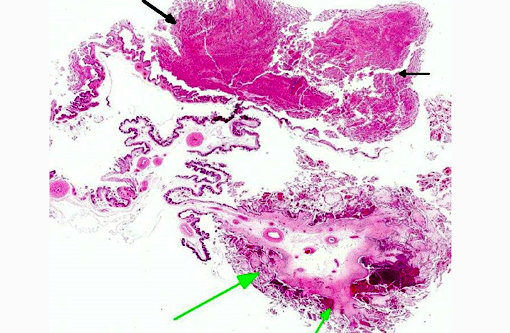
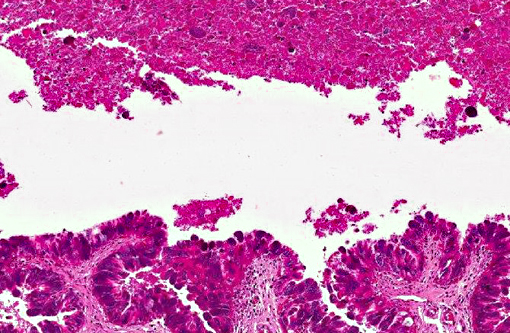
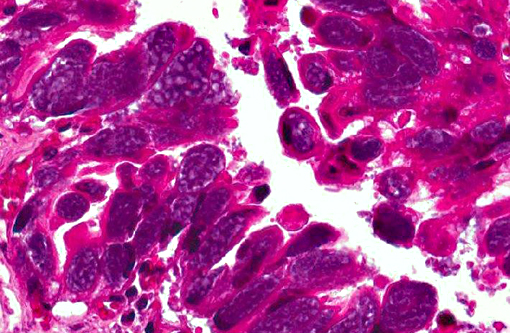
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
1. The detection of Coxiella burnetii by PCR with the primer pair of Trans1/Trans2 (687 bp) (Houver et al., 1992) and by nested PCR with the primer pairs of OMP1/OMP2 (501 bp) and OMP3/OMP4 (438 bp) (Zhang GQ et al., 1998) was employed for the final diagnosis of this case.
2. Other laboratory tests including chlamydiosis by PCR, foot & mouth disease by ELISA, and bluetongue by ELISA were all negative. Bacterial culture of the fetal tissues was also negative.
Condition:
Contributor Comment:
Coxiella burnetii is a potential bioterrorism and occupational hazardous agent. Q fever has been described worldwide except in Antarctica and New Zealand. Through the air-borne, it can be inhaled by humans. A single organism of C. burnetii may cause disease in a susceptible person. In animals, C. burnetii can infect many animal species, including domestic animals, birds, reptile, wildlife, and arthropods such as ticks. Cats and dogs may represent reservoirs of C. burnetii. Dogs may be infected by tick bites, by consumption of placentas or milk from infected ruminants, and by aerosol. The possibility of human Q fever acquired from infected dogs and cats has been reported. The infected animals are generally asymptomatic, but in mammals they may induce pneumonia, abortion, stillbirth, and delivery of weak lambs, calves or kids. The Coxiella burnetii-infected herds of cows have showed shedding the organisms within the milk for 13 months. The ewe can shed the organisms within the vaginal mucus for 71 days. People who may come into contact with infected animals are at the greatest risk, including farmers, slaughterhouse workers, laboratory workers, and veterinarians. In humans, C. burnetii causes highly variable clinical manifestations, ranging from acute to fatal chronic infections. However, about 60% of the human infections are asymptomatic seroconversions. Acute Q fever in humans displays mainly flu-like symptoms, atypical pneumonia or granulomatous hepatitis. Various rare clinical signs of meningoencephalitis, endocarditis, pericarditis, pancreatitis, and abortion have also been described. It is prudent for pregnant women to limit the contact with infected animals, especially with fetal fluids and unpasteurized milk.
In Taiwan, the first case of acute human C. burnetii infection was reported in 1993. Since 2005, samples have been routinely collected from goats, sheep, cattle, and wildlife for the study of the seroprevalence and histopathological changes of Q fever and the DNA sequence of C. burnetii. The results indicate that Q fever should be considered as a possible pathogen in association with the commonly observed abortion in goats, cattle, and wildlife in Taiwan. To our knowledge, this is the first diagnosis of Coxiella burnetii infection in Taiwan livestock.
JPC Diagnosis:
Conference Comment:
Although Coxiella burnettis zoonotic potential earns it the distinction of being an abortion agent of great importance, there are many other infectious causes of abortion outbreaks in ruminants which may induce similar placental lesions. Conference participants discussed other differentials for this case, including bacteria: Chlamydophila abortus, Histophilus somni, Yersinia pseudotuberculosis, Salmonella spp., Trueperella pyogenes, Leptospira spp., Listeria monocytogenes, Campylobacter spp., and Brucella spp., parasites: Toxoplasma gondii, Neospora caninum, Sarcocystis spp., and Tritrichomonas foetus, and viruses: Border disease virus, Wesselsbron, Bluetongue, Bovine herpesvirus 1, and several Bunyaviruses. Some are easily differentiated based on lesion distribution in the placenta, such as T. gondii and Y. pseudotuberculosis which both exclusively target the cotyledons.(9)
C. burnetti is known to infect many species and has recently been described as an emerging infectious disease in birds, in which it exhibits variable tissue tropism, with the most recent reports identifying myocarditis and hepatitis in a parrot.(10) Ectoparasites such as ticks are often cited as important vectors of transmission; however, intranasal inoculation was determined to be most effective in causing disease in pregnant goats.(8) Trophoblasts in the allantochorion of the placenta are the primary target of infection and replication of the bacteria; and their main excretion route from these infected goats is during delivery of the aborted fetus, as bacterial DNA was not detected in feces or vaginal mucus in a recent study.(8)
C. burnetti is considered highly infectious, though its inoculation does not necessarily translate into reproductive disease. A recent publication identified the organism in 75% of abortion submissions based on PCR, but when combined with histopathology and additional ancillary testing, C. burnetti was only determined to be the actual cause in 21% of those cases. The more commonly identified cause of abortions in this study was T. gondii, with Campylobacter spp. and Chlamydophila spp. also being more prevalent than the agent of Q fever.(4)
References:
1. Bacha WJ, Bacha LM. Color Atlas of Veterinary Histology. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2000:222.
2. Berri M, Crochet D, Santiago S, Rodolakis A. Spread of Coxiella burnetii infection in a flock of sheep after an episode of Q fever. Vet Rec. 2005:157, 737-740.
3. Glazunova O, Roux V, Freylikman O, Sekeyova Z, Fournous G, Tyczka J, et al. Coxiella burnetii genotyping. Emerg Infect Dis. 2005;11, 1211-1217.
4. Hazlett MJ, McDowall R, DeLay J, et al. A prospective study of sheep and goat abortion using real-time polymerase chain reaction and cut point estimation shows Coxiella burnetii and Chlamydophila abortus infection concurrently with other major pathogens. J Vet Diagn Invest. 2013;25(3):359-368.
5. Lai CH, Huang CK, Chin C, Chung HC, Huang WS, Lin CW. Acute Q fever: An emergin and endemic disease in Southern Taiwan. Scand J Infect Dis. 2008;40:105-110.
6. Raoult D, Drancourt M, Vestris G. Bactericidal effect of doxycycline associated with lysosomtropic agents on Coxiella burnetii in P388D1 cells. Antimicrob Agents Chemother. 1990;34:1512-1514.
7. Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis 2005;5:219-226.
8. Roest HJ, Gelderen BV, Dinkla A, et. al. Q fever in pregnant goats: pathogenesis and excretion of coxiella burnetii. PLoS One 2012;7(11):1-14.
9. Schlafer DH, Miller RB. Female genital system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol. 3. 5th ed. Philadelphia, PA: Elsevier Saunders; 2007:484-530.
10. Vapniarsky N, Barr BC, Murphy B. Systemic Coxiella-like infection with myocarditis and hepatitis in an eclectus parrot (Eclectus roratus). Vet Pathol. 2012;49(4):717-722.
11. Woldehiwet Z. Q fever (coxiellosis): epidemiology and pathogenesis. Res Vet Sci. 2004;77:93100.