WSC 22-23
Conference 3
CASE I:
Signalment:
2-year-old, male, C57/BL6 CuZnSOD wild type (Mus musculus)
History:
This mouse recently arrived at the facility and was housed in quarantine. Two days after arrival the mouse presented clinically with abdominal distension. On physical examination, a large abdominal mass was palpated, and humane euthanasia was elected.
Gross Pathology:
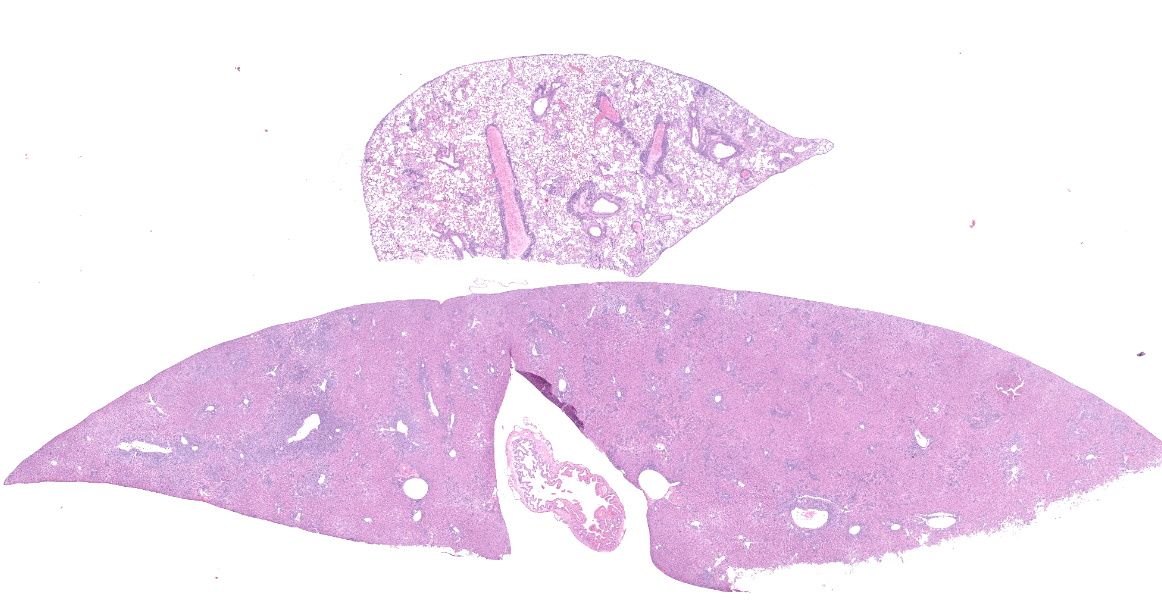
The mouse was in fair body condition (BCS 2.5/5). The lungs were mottled with patchy pale yellow to pink foci. Within the abdomen, there were three encapsulated masses associated with the mesentery that ranged in size from 0.5-2cm in diameter (gross photo, black asterisks). On cut section, the masses were abscessed. The liver and the spleen were diffusely enlarged.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
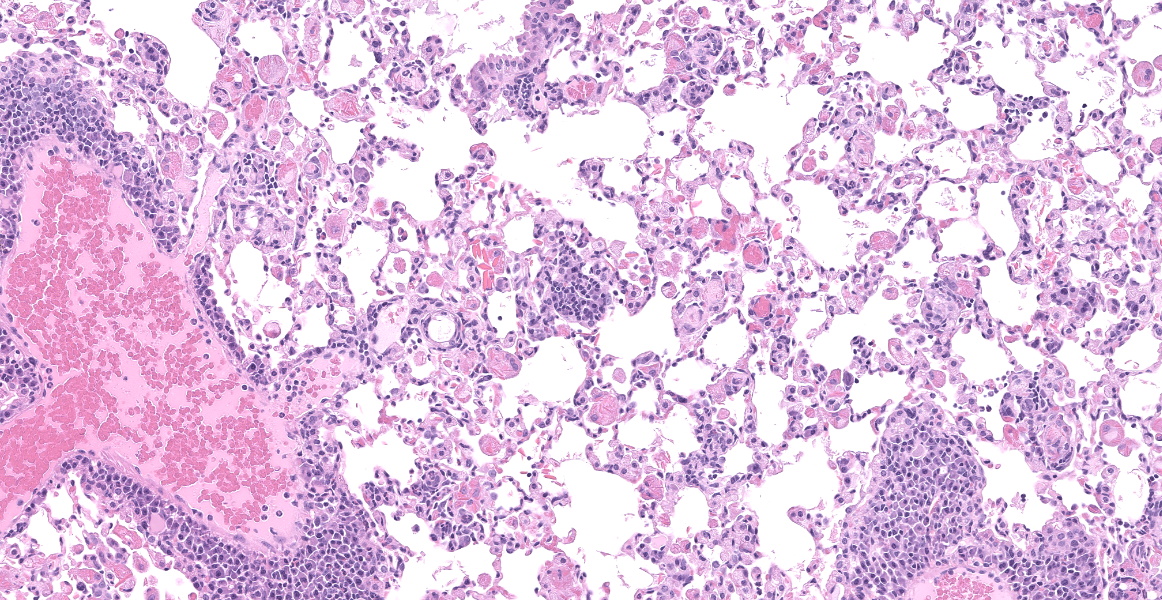
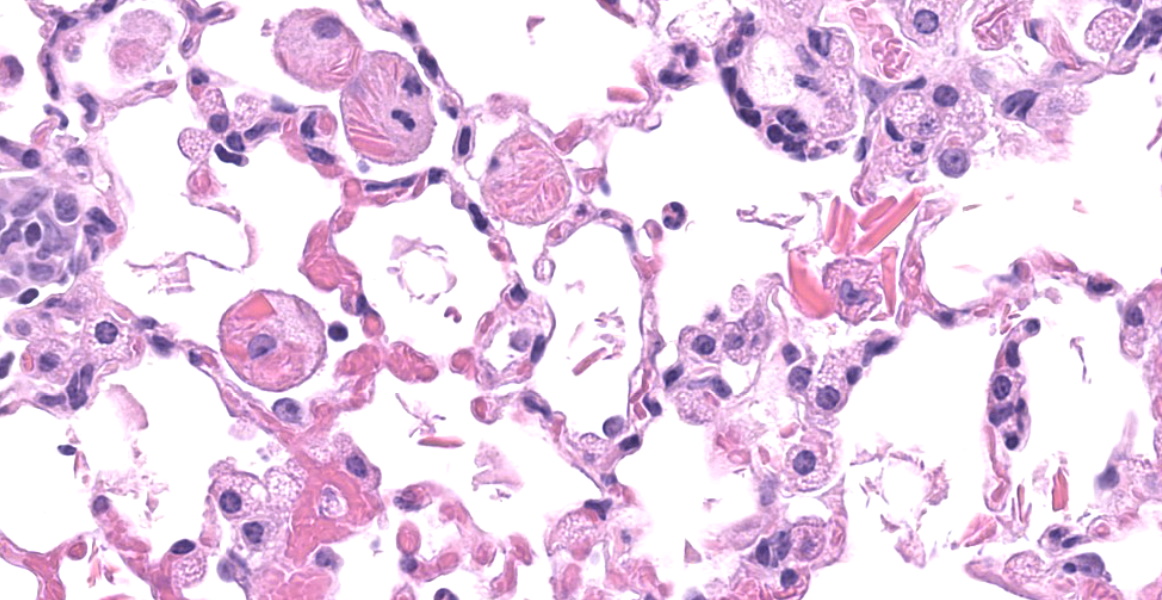
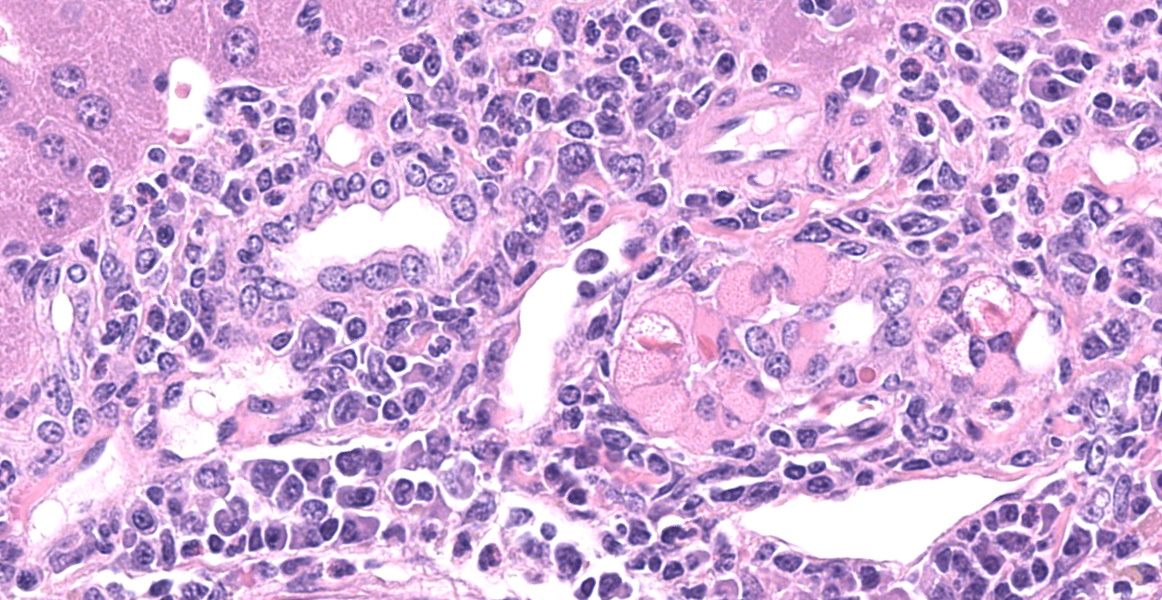
Throughout all lung lobes there are multifocal to coalescing inflammatory infiltrates filling alveolar spaces. Inflammatory infiltrates are primarily composed of large plump macrophages and multinucleated giant cells admixed with eosinophils and fewer lymphocytes, plasma cells, and neutrophils. Macrophages and multinucleated giant cells contain abundant intracytoplasmic brightly eosinophilic partially refractile acicular to rectangular crystals admixed with homogenous eosinophilic hyalinized material. Crystals and hyaline are also present within in the extracellular space. There are perivascular and peribronchiolar lymphoplasmacytic cuffs.
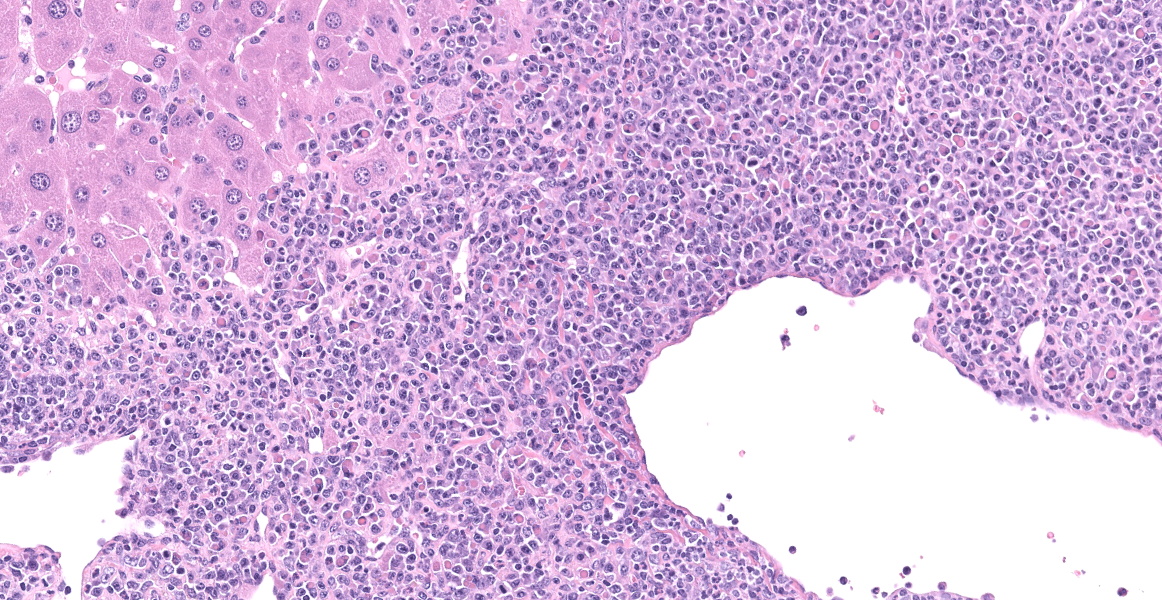
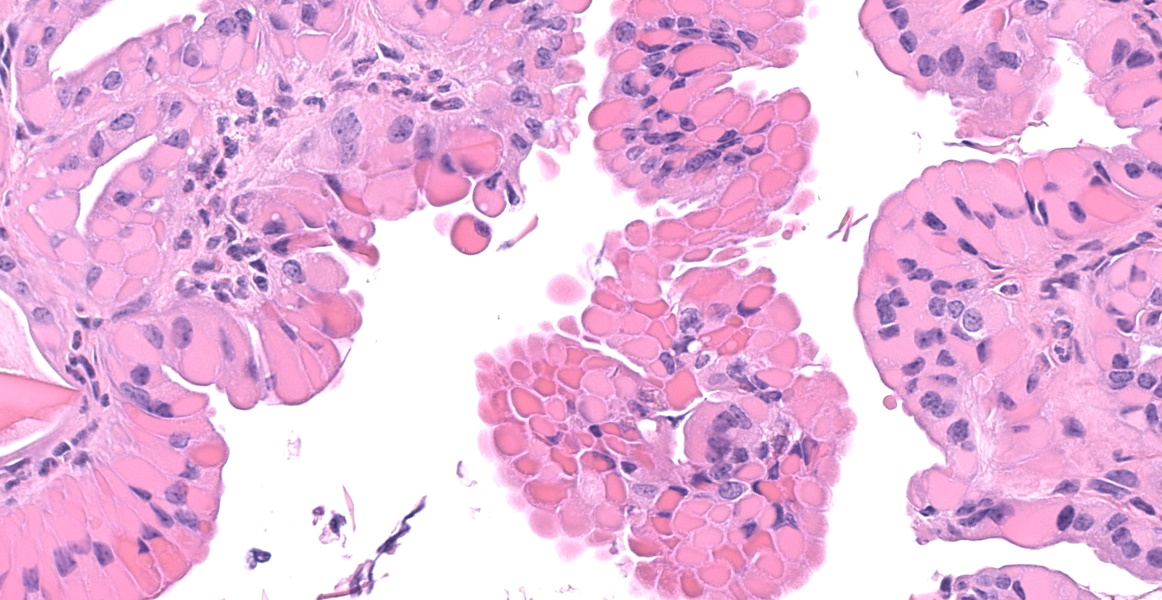
In the section of liver, there is marked proliferation of myeloid precursors primarily surrounding portal regions. Myeloid precursors infiltrate into the parenchyma and bridge adjacent portal tracts. Hepatic cords are attenuated. The biliary and gall bladder epithelium are expanded by intracytoplasmic eosinophilic hyalinized material. There are acicular and rectangular crystals within the gall bladder.
Contributor's Morphologic Diagnoses:
Lung: Eosinophilic and granulomatous pneumonia, chronic, severe, with eosinophilic crystals.
Liver and spleen: Myeloid hyperplasia, marked, diffuse.
Bile ducts/gall bladder: Epithelial hyalinosis, marked, multifocal.
Contributor's Comment:
Eosinophilic crystalline pneumonia (ECP), also known as acidophilic macrophage pneumonia, is a common age-related background lesion in C57/BL6 and 129/Sv mice and in many of their knockout and transgenic derivatives.5,7,9 This lesion can be exacerbated during concurrent pulmonary disease, and has been reported in association with systemic infectious, neoplastic, hypersensitivity, and lymphoproliferative disorders.5 Eosinophilic crystals are derived from macrophages and are primarily composed of iron, alpha-1 antitrypsin, immunoglobulin, and granulocyte breakdown products.2,6 In addition to accumulation within macrophages and the lung, this protein can also accumulate within epithelial cells of the pancreas, stomach, liver and gallbladder, and the olfactory epithelium.2,4 Of note, in addition to ECP and epithelial hyalinosis, this mouse also had multiple abdominal abscesses and myeloid hyperplasia in the spleen, kidney, and liver. Thus, it was determined that the severe ECP in this case was likely due to age and exacerbated by chronic systemic inflammation.
Contributing Institution:
In Vivo Animal Core, University of Michigan, Unit for Laboratory Animal Medicine
https://animalcare.umich.edu/business-services/vivo-animal-core
ulam-ivac@umich.edu
JPC Diagnosis:
- Lung: Alveolitis, granulomatous, diffuse, moderate, with intrahistiocytic and extracellular eosinophilic crystals and perivascular plasmacytosis.
- Liver, gallbladder: Biliary epithelial hyalinosis and extracellular crystals, diffuse, severe.
- Liver: Extramedullary hematopoiesis, diffuse, severe.
JPC Comment:
As the contributor mentions, eosinophilic crystalline pneumonia occurs regularly in aged B6 mice and has a more rapid onset in the moth-eaten phenotype of B6 mice.2 Moth-eaten strains have a deficiency of Shp1, a protein tyrosine phosphatase involved in the immune signaling pathways of multiple hematopoietic cell types, due to a spontaneous mutation of Ptpn6.1 In the lung, Shp1 deficiency is associated with the rapid accumulation of eosinophilic crystals within pulmonary macrophages, while in the skin, Shp1 deficiency results in neutrophilic inflammation and dendritic-cell driven autoimmunity producing chronic dermatitis and alopecia, the basis of the 'moth-eaten' designation.1,10 Other strains which are predisposed to hyalinosis include female CYP1A2-null mice and female 129S4/SvJae mice, with one study documenting hyalinosis of the glandular stomach in over 95% and 45% of these strains, respectively.10 These mice had grossly visible plaque-like lesions in the cardia; histologically, there was disorganization, hyperplasia, and hyalinization of gastric epithelial cells and abundant extracellular eosinophilic crystals.10
Grossly, ECP causes firm pale tan lesions in the lung which fail to collapse.8 Affected gallbladders may have mural thickening and opacification with bile duct fibrosis.10
In addition to the components listed by the contributor, eosinophilic crystals contain Ym1 (eosinophilic chemotactic factor) and Ym2, two closely related chitinases with different patterns of distribution throughout the body.2,10 Pulmonary lesions primarily contain Ym1, and other organ systems contain a mixture of both Ym1 and Ym2.2
Differential diagnoses for ECP include pulmonary histiocytosis, which typically occurs in the subpleural regions, or alveolar lipoproteinosis, may contain granular eosinophilic material (surfactant) and scattered macrophages.2 Hemorrhage can lead to extracellular hemoglobin crystal formation.2,3 Eosinophilic crystals have also been seen in particulate inhalation studies, Cryptococcus neoformans infections in mice, and some dietary toxicity and drug studies in rats.3
References:
- Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity. 2013;38: 489-501.
- Barthold SW, Griffey SM, Percy DH. Pathology of Laboratory Rodents and Rabbits. 4th Ames, IO: Wiley Blackwell; 2016: 3,94-95,100.
- Cesta MF, Dixon D, Herbert RA, Staska LM. Lung - Crystals. US Department of Health and Human Services National Toxicology Program Nonneoplastic Lesion Atlas. Accessed 11 August 2022. https://ntp.niehs.nih.gov/nnl/respiratory/lung/crystal/index.htm
- Giannetti N, Moyse E, Ducray A, et al. Accumulation of Ym1/2 protein in the mouse olfactory epithelium during regeneration and aging. Neuroscience. 2004;123: 907-917.
- Hoenerhoff MJ, Starost MF, Ward JM. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Vet Pathol. 2006;43(5).
- Klug JJ, Snyder JM. Eosinophilic crystalline pneumonia, an age-related lesion in mice. Aging Pathobiol Ther. 2020;2: 232-233.
- Murray AB, Luz A. Acidophilic Macrophage Pneumonia in Laboratory Mice. Vet Pathol. 1990;27(4).
- Pettan-Brewer C, Treuting PM. Practical pathology of aging mice. Pathobiol Aging Age Relat Dis. 2011;1.
- Radaelli E, Castiglioni V, Recordati C, et al. The Pathology of Aging 129S6/SvEvTac Mice. Vet Pathol. 2016;53(2): 477-492.
- Ward JM, Yoon M, Anver MA, et al. Hyalinosis and Ym1/Ym2 Gene Expression in the Stomach and Respiratory Tract of 129S4/SvJae and Wild-Type and CYP1A2-Null B6,129 Mice. Am J Pathol. 2001; 158(1):323-332.
- Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. 2020;5: 201.