Signalment:
Six-year-old, castrated male, boxer, (
Canis familiaris).The
dog presented with a 20-day history of seizure-like activity (lateral
recumbency, drooling, and paddling) with increasing frequency. Phenobarbital
administration controlled the symptoms for several weeks, but the dog gradually
became lethargic and inappetant with head twitching behavior. The owners
elected euthanasia due to concern about the dogs quality of life.
Gross Description:
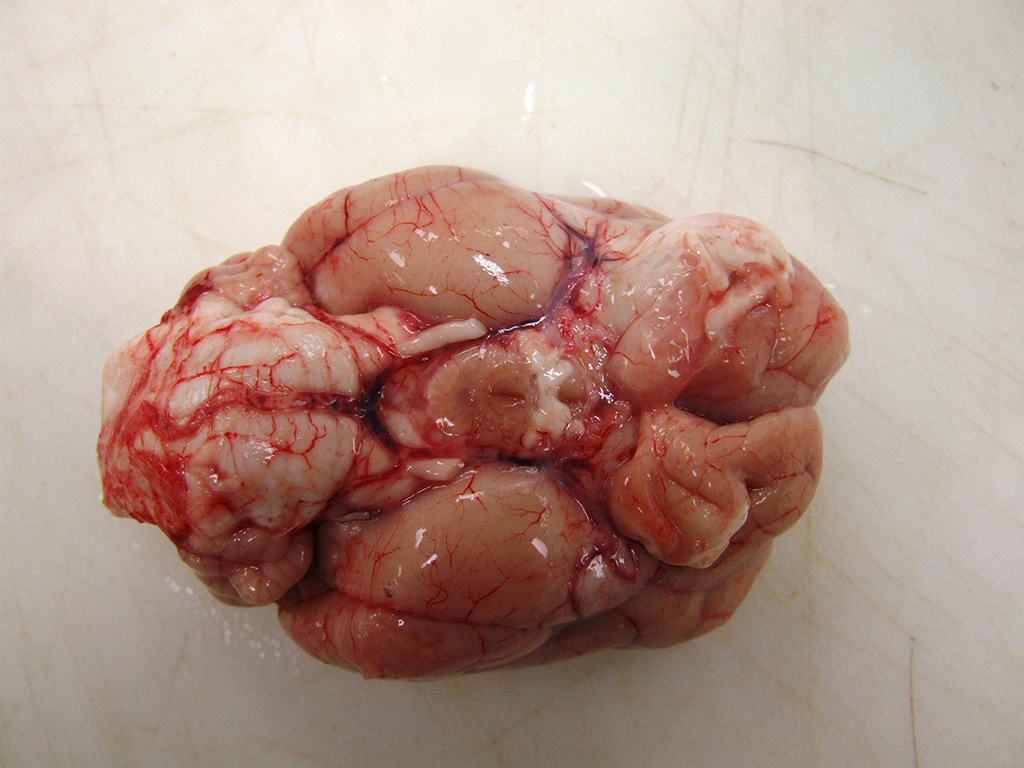
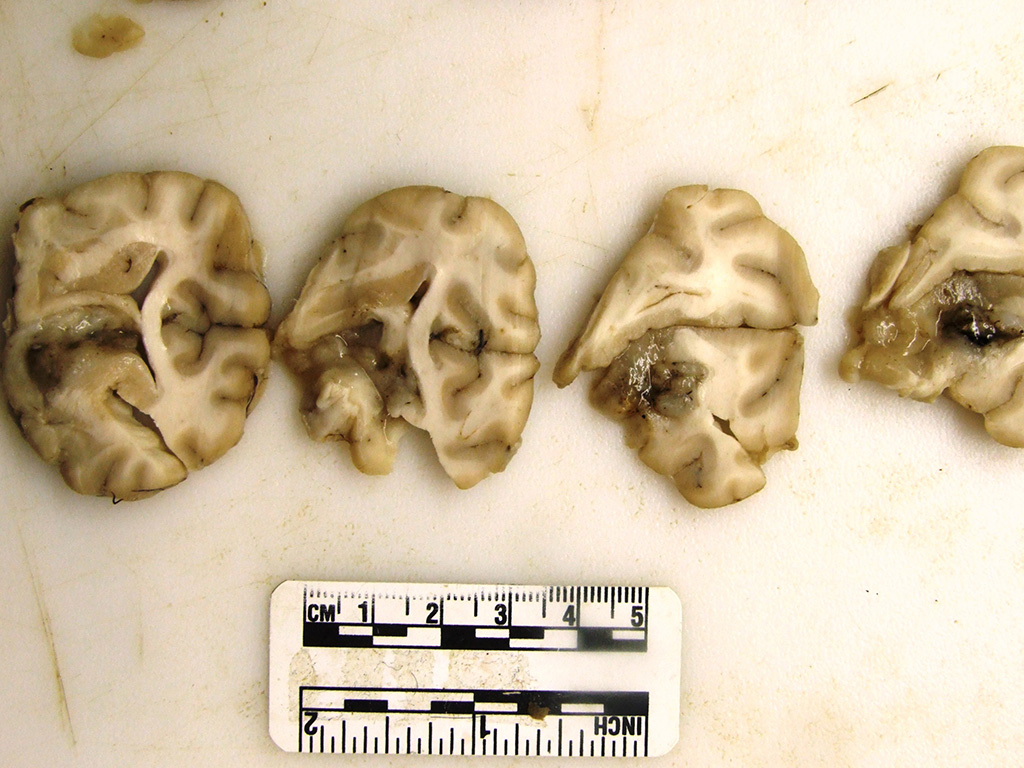
The
rostral aspect of the right frontal lobe of the brain was enlarged, with
shallow sulci and flattened gyri, and was deviated left of the midline. The
brain was fixed in formalin and sectioned for examination. The cranioventral
portion of the right cerebral hemisphere contained a gray, translucent,
gelatinous, 2cm x 2cm x 4cm mass, extending from the frontal lobe of the
cerebrum into the mesencephalon.
Histopathologic Description:
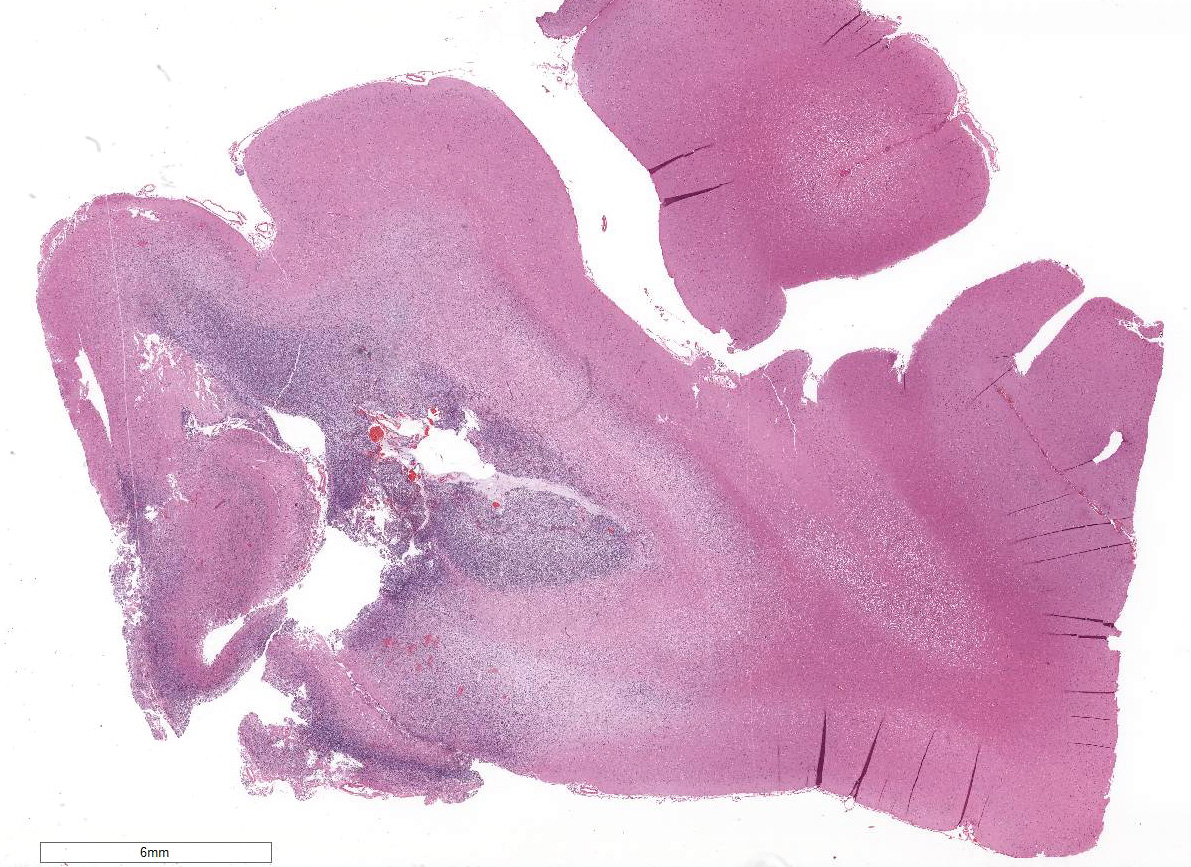
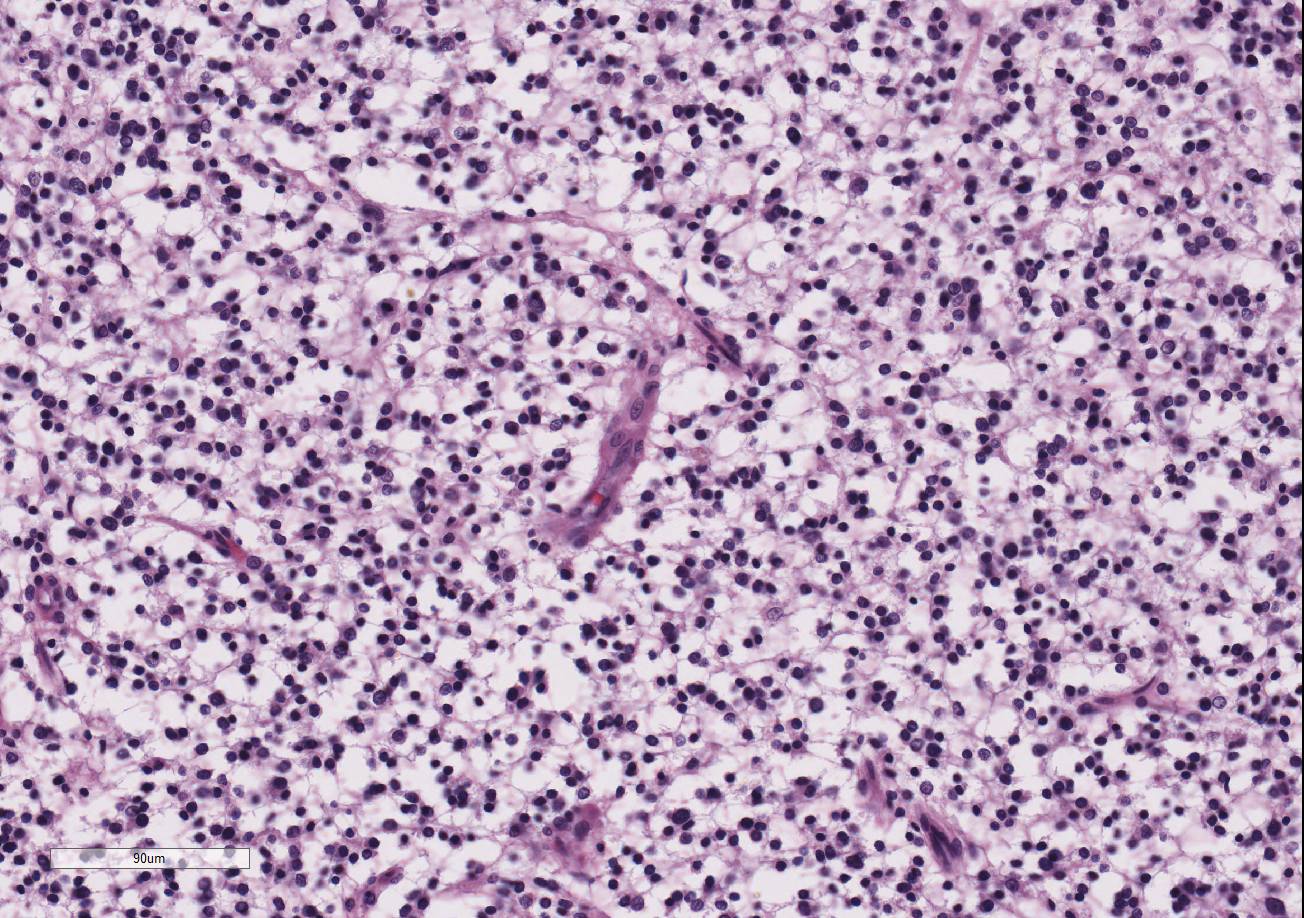
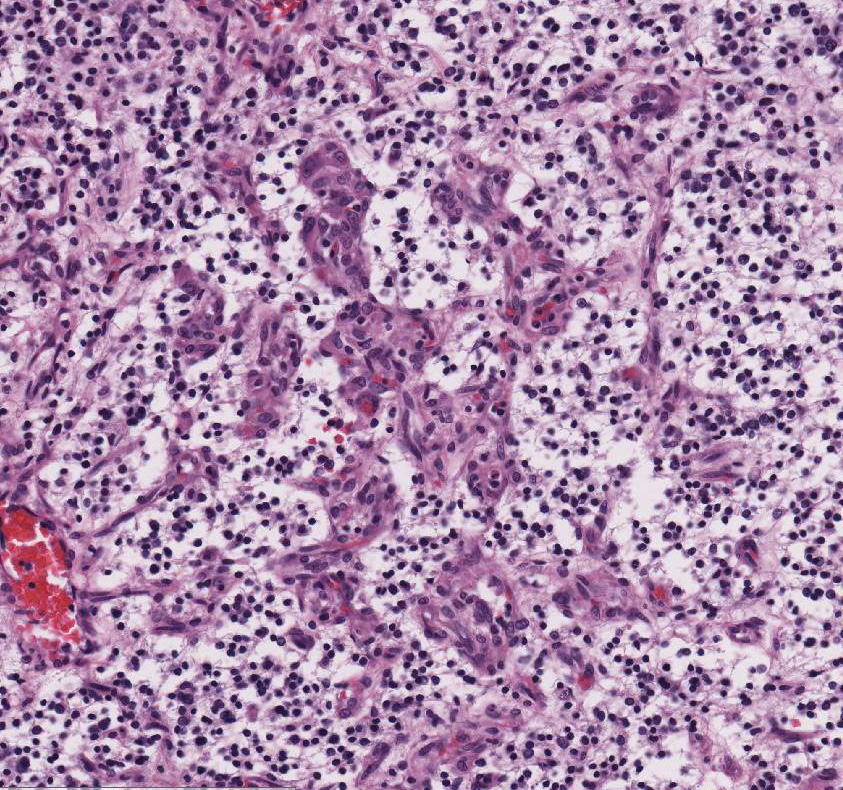
Within the white matter of the
cerebral cortex, there is a poorly demarcated, unencapsulated, in-filtrative
neoplastic mass. The neoplasm is composed of round to polygonal cells arranged
as a loose meshwork or occasionally as closely packed sheets with a honeycomb
appearance and scant fibro-vascular stroma. Neoplastic cells are 10-14 microns
in diameter and have distinct cell borders; scant to moderate amounts of
eosinophilic, fibrillar cytoplasm; a prominent perinuclear clear zone (peri-nuclear
halo); and small, irregularly round, hyperchromatic nuclei with indistinct
nucleoli. The mitotic rate is less than one per ten 400x high power fields.
Capillary blood vessels within the mass are prominent with marked endothelial
hypertrophy. Other variable features that are present in some slides: scattered
foci of hemorrhage, glomeruloid microvascular proliferation, and pseudocystic
cavities containing homo-geneous, lightly basophilic (mucinous) material. Neoplastic cells were negative for GFAP on immunohistochemical staining.
Morphologic Diagnosis:
Brain (cerebrum): Oligodendroglioma.
Lab Results:
Bloodwork was unremarkable.
Condition:
Oligodendroglioma
Contributor Comment:
This
case had a classic signalment and presentation for canine oligodendrogliomas.
Oligo-dendrogliomas occur most commonly in dogs, particularly in older animals
and brachycephalic breeds (boxers, Boston terriers, and bulldogs), and rarely
in other species including humans, cats, cattle, and horses.
1,2,9 In
a study involving 173 dogs, it was found that dogs with oligo-dendrogliomas are
3.6 times more likely to have seizures compared with dogs with other types of
primary brain tumors.
9 Other common presenting clinical complaints
include mentation change, vision loss, neck pain, and vestibular syndrome.
9
Bloodwork is usually unremarkable, as it was in this case. The most
frequent location for oligo-dendrogliomas is the white or gray matter of the
cerebral hemispheres, particularly the olfactory area and rostral lobes, but
can occur
caudally or in the spinal cord.
5,9 Grossly, they appear as large,
soft, gelatinous or mucoid, well-demarcated masses with grayish-blue matrix and
gray to pink stroma on cut section.
1,2,5 Histologically,
oligodendrogliomas are moderately to highly cellular, and characterized by
dense sheets of uniform cells.
1,5 Delayed fixation causes an arti-factual
"honeycomb" cell pattern with a perinuclear halo effect.
1,2,5
Prominent microvascular proliferation is common, often with formation of a
delicate "chicken-wire" pattern or vascular loops with
glomerular-like tufts, as seen in this case.
1,2,5 Foci of
hemorrhage, mineralization, or microcystic areas containing blue-staining
mucinous material may also be present.
1,2,5 Tumors can extend along
or through the leptomeninges or ependymal surfaces; intraventricular growth may
be associated with widespread intraventricular metastases.
5 Anaplastic
oligodendrogliomas are characterized by focal or diffuse anaplasia with
prominent proliferation of glomeruloid vessels at the tumor margins, nuclear
polymorphism, and increased mitotic index (1-2 per HPF), necrosis, and/or
meningeal infiltration.
1 Intermingled astrocytes are common, and in
some tumors polymorphic multinucleated giant cells may be numerous.
1
Often, areas of necrosis with peripheral glial cell palisading can be found.
5
Due to the lack of malignant features, in this case, the tumor was diagnosed as
a benign oligodendroglioma.
Oligoastrocytomas
are rare mixed glial tumors composed of neoplastic astrocytes and
oligodendroglia, in which the two cell populations may be diffusely
intermingled or in geographically distinct clusters.
1,2,5 Canine
oligoastrocytomas are predominantly composed of oligodendroglial cells with at
least 25-30% neoplastic astrocytes; lower percentages of astrocytic elements
are interpreted as reactive proliferating cells within oligodendrogliomas.
1,5
Anaplastic (malignant) oligoastrocytomas are characterized by increased
cellularity, nuclear atypia, high mitotic activity, vascular proliferation, and
necrosis, and may be difficult to differentiate from high-grade astrocytomas.
1
Ultrastructurally,
oligodendrogliomas have no obvious distinguishing features, appearing as cells
with sparse microtubules and few organelles in the cytoplasm and frequent
desmosomal junctions between cells.
5
Historically,
diagnosis of oligo-dendrogliomas has relied on gross and microscopic tumor
morphology and negative GFAP staining of cells, due to lack of specific
markers.
2,4,5 In recent years, several markers have been
investigated, including doublecortin, olig2, and CNPase.
3,4
Doublecortin is a cytoplasmic neuronal precursor marker which is frequently
expressed in oligodendrogliomas and embryonal neoplasms such as neuro-blastomas
and PNETs, but infrequently expressed in astrocytomas.
3 Olig2 is a
nuclear transcription factor that is required for oligodendrocyte
differentiation but not astrocyte development, and has been shown to stain all
oligodendrogliomas in one study.
4 Non-neoplastic astrocytes do not
stain with Olig2; however, astrocytomas can have positive nuclear staining.
4
CNPase is a cytoplasmic phosphodiesterase that is expressed early in
myelination.
4 It stains normal and neoplastic oligodendrocytes, but
weak cytoplasmic staining of canine astro-cytomas can also occur.
4 A
combination of negative GFAP staining and positive Olig2 staining may be most
helpful in identification of oligodendrogliomas. Positive staining for factor
VIII-like antigen and smooth muscle actin highlights the neoplasm's
characteristic microvascular pro-liferations.
5 In our case, the
neoplasm stained negatively for GFAP. No staining for the other potential
oligodendroglioma markers was attempted.
JPC Diagnosis:
Brain, cerebrum: Oligo-dendroglioma, boxer,
Canis familiaris.
Conference Comment:
Despite
some moderate slide variability, the contributor provides a great example and
com-prehensive review of oligodendrogliomas in the canine central nervous
system (CNS). Oligo-dendrogliomas are the third most common primary neoplasm in
the dog brain, after meningiomas and astrocytomas.
6 These soft
gelatinous neoplasms typically arise in the telencephalic or diencephalic
cerebral white matter, but can uncommonly occur in the brainstem, spinal cord,
or within the ventricular system as a single tumor or as multiple concurrent
oligodendrogliomas as a result of metastasis through the cerebro-spinal fluid.
2,6,8
Oligodendrogliomas
originate from oligo-dendrocytes, which normally function
to provide support and myelination to axons within the white
matter tracts of the CNS.
7 Each oligodendrocyte can envelop up to 50
axons based on the thickness of the myelin sheath needed. Oligodendrocytes also
serve a role in the production of neurotropic factors important for
the remyelination of demyelinated axons in the CNS.
7 Schwann cells
provide an equivalent function in the peripheral nervous system (PNS); however,
unlike oligodendrocytes, Schwann cells can only envelope one part of a single
axon. Schwann cells are also extremely important in axonal regeneration in the
PNS.
10
As mentioned by the
contributor, positive immunohistochemical staining for oligo-dendrocyte
transcription factor-2 (Olig2) in combination with negative glial fibrillary
acidic protein (GFAP) has been shown to be an extremely useful tool in the
diagnosis of oligodendrogliomas. The proportion of Olig2-positive cells has
been shown to be significantly higher in oligodendrogliomas when compared with
other glial tumors such as astrocytoma and oligoastrocytoma, mentioned above.
4,6
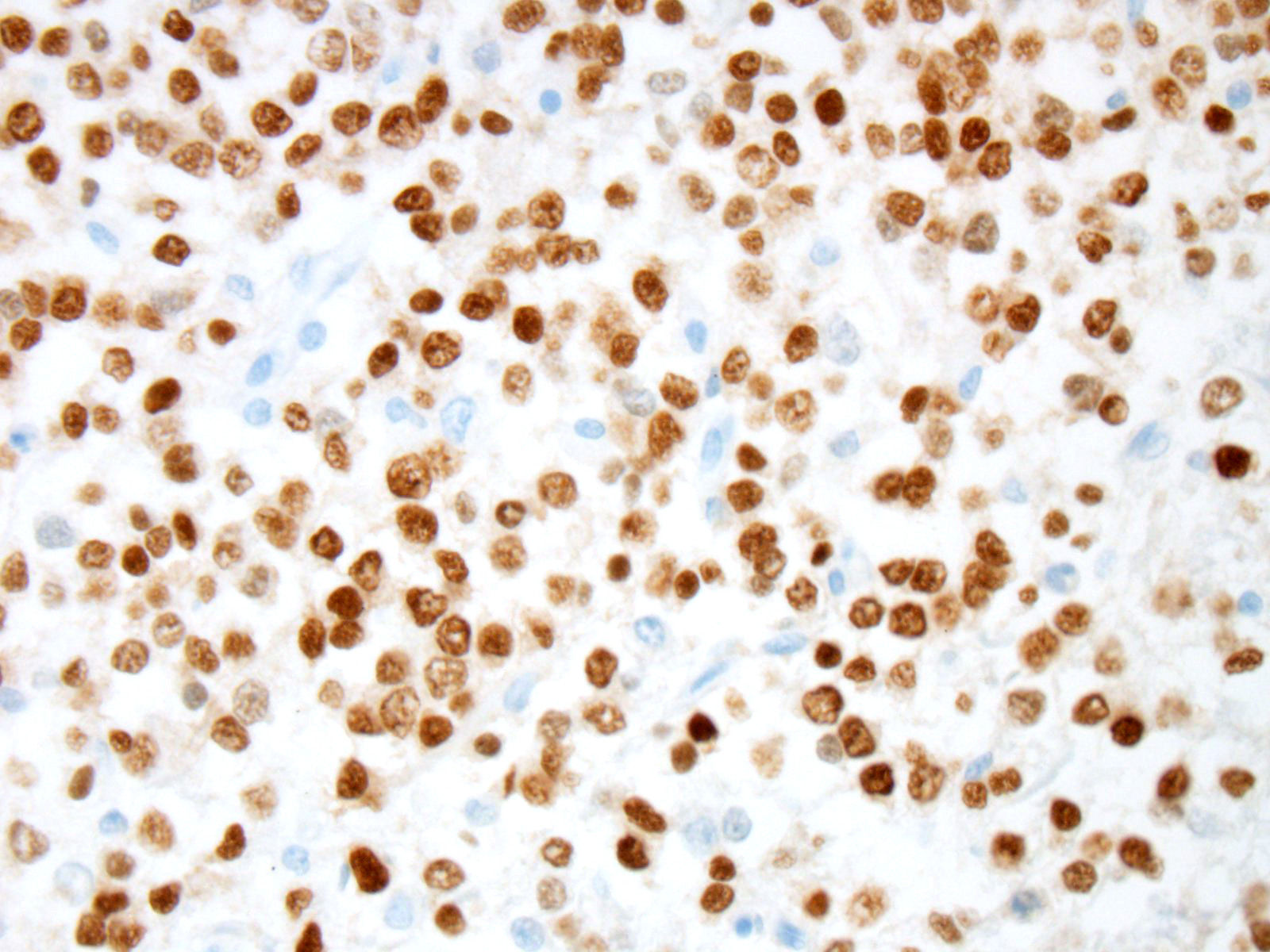
Prior to the conference, an Olig2 and GFAP stains were performed by the Joint
Pathology center. In this case, neoplastic cells showed strong and diffuse
intranuclear immunoreactivity to Olig2. Expression of intranuclear Olig2 immuno-reactivity
is restricted to the neoplasm and resident oligodendrocytes within the
unaffected section of cerebrum. Additionally, neoplastic cells are diffusely
immunonegative for GFAP. The GFAP-positive cells noted by conference
participants at the periphery and within the neoplasm are interpreted as
reactive astrocytes, which can occur due to tumor-induced activation of the residential astroglial network.
This staining pattern is consistent with the contributors diagnosis of an
oligodendroglioma, in this case.
2,4,6,8
References:
1. Burger
PC, Scheithauer BW: Tumors of neuroglia and choroid plexus. In:
AFIP Atlas
of Tumor Pathology, Series 4, Tumors of the Central Nervous System. ed.
Silverberg SG. Washington DC: ARP Press; 2007:225-233.
2. Cantile
C, Youssef S. Nervous system. Maxie MG ed. In:
Jubb Kennedy and Palmer's
Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA:
Elsevier Saunders; 2016:400.
3. Ide,
Uchida K, Kikuta F, Suzuki K, Nakayama H. Immunohistochemical characterization
of canine neuro-epithelial tumors.
Vet Pathol. 2010; 47(4)741-750.
4. Johnson
GC, Coates JR, Wininger F. Diagnostic immunohistochemistry of canine and feline
intracalvarial tumors in the age of brain biopsies.
Vet Pathol. 2014;
51(1)146-160.
5. Koestner
A, Higgins RJ. Tumors of the nervous system. In:
Tumors in Domestic Animals.
ed. Meuten DJ. 4th ed. Ames, IA: Iowa State Press; 2002:703-706.
6. Kovi
RC, Wünschmann A, Armién AG, Hall K, Carlson T, Shivers J, Oglesbee MJ. Spinal meningeal
oligo-dendrogliomatosis in two boxer dogs.
Vet Pathol. 2013;
50(5):761-764.
7. Kremer
D, Gottle P. et al. Pushing forward: Remylination as the new frontier in CNS
disease.
Trends Neurosci. 2016; 39(4):246-263.
8. Rissi
DR, Levine JM, et al. Cerebral oligodendroglioma mimicking intra-ventricular
neoplasia in three dogs.
J Vet Diagn Invest. 2015; 27(3):396-400.
9. Snyder JM,
Shofer FS, Van Winkle TJ, Massicotte C: Canine intracranial primary neoplasia:
173 cases (1986-2003).
J Vet Intern Med. 2006; 20:669-675.
10. Toy
D, Namgung U. Role of glial cells in axonal regeneration.
Exp neurobiol.
2013; 22:68-76.