Signalment:
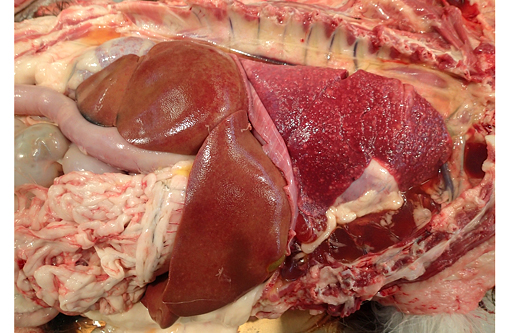
At presentation the dog had generalized heart sounds; radiographs showed a diffuse, mixed, predominantly interstitial pattern in all lung lobes. Transtracheal wash revealed neutrophilic inflammation with protozoa. The dog deteriorated progressively and spontaneously arrested.
Gross Description:
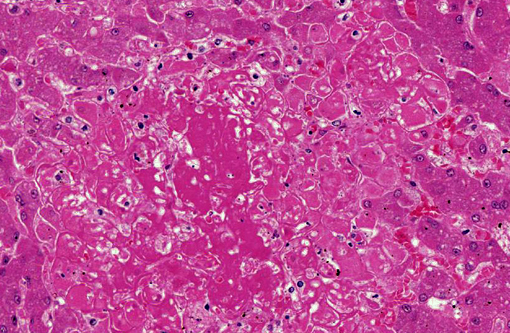
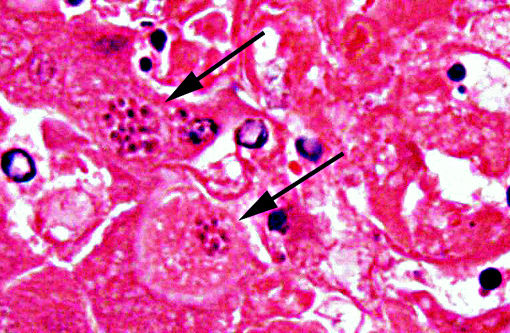
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Infection occurs by ingestion of sporulated oocysts excreted in the feces of felids, by ingestion of tissues of intermediate hosts that contain encysted bradyzoites or tachyzoites, and less frequently by vertical transmission. Once ingested, sporozoites excyst and multiply in the intestinal epithelial cells as tachyzoites. Tachyzoites can either disseminate and infect cells throughout the body resulting in the necrosis and less commonly non-suppurative inflammation characteristic of toxoplasmosis, or encyst in tissues as bradyzoites. Following ingestion of tissue cysts by an intermediate host, bradyzoites will excyst, become tachyzoites, and the cycle continues.(1) Necrosis is caused by rupture of infected cell membranes by rapidly dividing tachyzoites. Inflammation is typically not associated with the cysts and can be minimal in association with the tachyzoites.(2) Tissue cysts are presumed to persist throughout the life of the host. Even though a high percentage of animals are serologically positive for toxoplasmosis, only a few animals develop clinical disease. Immunosuppression commonly causes reactivation of latent infections caused by T. gondii and is usually characterized by involvement of the central nervous system, occasionally the lungs and infrequently other organs, including the skin.(4)
The tachyzoites, bradizoites and tissue cysts of Neospora caninum and Toxoplasma gondii appear essentially identical by standard light microscopy. Immunohistochemistry and PCR are commonly used to confirm the diagnosis. Even though IHC using polyclonal antibodies against T. gondii is considered sensitive, positive results might not be conclusive, due to moderately low specificity of polyclonal antibodies that can cross-react between T. gondii and N. caninum.(5)
Ultrastructure is also used to distinguish between T.gondii and N.caninum. Some of the most prominent ultrastructural differences occur in the number, appearance and location of rhoptries, looped-back rhoptries, micronemes, dense granules, small dense granules and micropores. The tissue cysts of both parasites are basically similar, being surrounded by a cyst wall and not compartmentalised by septa. The cyst wall of N. caninum is usually irregular and substantially thicker (<0.5 mm), than those of T. gondii which are typically smooth and 0.5 mm thick.(6)
JPC Diagnosis:
1. Liver: Hepatitis, necrotizing, random, multifocal, moderate, with edema and intrahepatocytic, intrahistiocytic, and extracellular zoites.
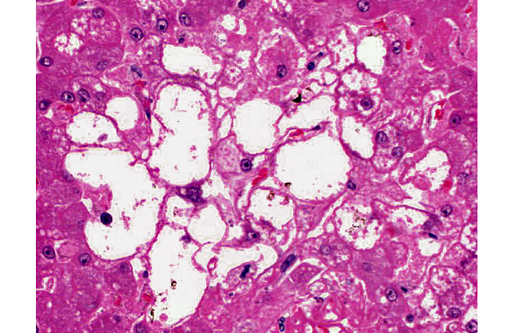
2. Liver, hepatocytes: Glycogenosis, centrilobular and midzonal, multifocal, moderate.
Conference Comment:
Toxoplasmosis is a significant contributor to abortion in domestic animals due to both cotyledonary and caruncular necrosis of the placenta. Other characteristic lesions include interstitial pneumonia, lymphadenitis, myocarditis, nonsuppurative meningitis, ophthalmitis and hepatic necrosis.(2)
T. gondii is an obligate intracellular parasite, and its growth and replication only occur in target cells and eventually cause cell death. At the core of its infectivity is the ability to cross barrier systems, including intestinal mucosa, the blood-brain barrier, the blood-retina barrier and the placenta. This process involves parasite motility, likely in the tachyzoite stage, and interactions between parasite adhesins and target cell receptors. The cellular binding occurs via the parasite surface proteins SAGs, which are expressed in abundance on tachyzoites, and SAG1 receptors in addition to laminin and lectin on target cells.(7)
The contributor accurately details the distinguishing characteristics between Toxoplasma and Neospora on electron microscopy. Both parasites induce similar lesions and appear identical with histopathology; however, Toxoplasma is always found within a parasitophorous vacuole while Neospora may not be. Other organisms which may be found within parasitophorous vacuoles include Sarcocystis, though these are primarily in skeletal or cardiac muscle.(2)
The glycogenosis noted in the hepatocytes is secondary to the administration of exogenous corticosteroids. Glycogenosis may be histologically differentiated from lipidosis as the vacuoles seen in glycogenosis are coalescing rather than discrete. In most cases, steroid-induced gluconeogenesis causes increases in both hepatocellular lipid and glycogen, but glycogensosis is often the predominant histologic change.
We favor the use of two morphologic diagnoses in this case, one for the protozoal infection and one for the glycogenosis, as they are part of two distinct, albeit related processes.
References:
1. Dubey JP, Lindsay DS, Lappin MR. Toxoplasmosis and other intestinal coccidial infections in cats and dogs. Vet Clin North Am Small Anim Pract. 2009;39:10091034.
2. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier Saunders; 2007:271-273.
3. Mayberry C, Maloney SK, Mitchell J, Mawson PR, Bencini R. Reproductive implications of exposure to Toxoplasma gondii and Neospora caninum in western grey kangaroos (Macropus fuliginosus ocydromus). 2014;50(2):364-368.
4. Rodrigues Hoffman A, Cadieu J, Kiupel M, Lim A, Bolin SR, Mansell J. Cutaneous toxoplasmosis in two dogs. J Vet Diagn Invest. 2012;24:636-640.
5. Kaufmann H, Yamage M, Roditi I, et al. Discrimination of Neospora caninum from Toxoplasma gondii and other apicomplexan parasites by hybridization and PCR. Mol Cell Probes. 1996;10:28929.
6. Speer CA, Dubey JP, McAllister MM, Blixt JA. Comparative ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum and Toxoplasma gondii. Int J Parasitol. 1999;29:1509-1519.
7. Zachary JF. Mechanisms of microbial infections. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:239-240.