Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 6
October 4th, 2017
CASE III: T (JPC 4035426).
Signalment: 12-month-old, female, breeder hen turkeys, Meleagris gallopavo, avian.
History: A spike in mortality was reported in this flock of 2200 breeder hens. Affected live birds were down, unable to rise, had drooped wings and were panting. In the tom barn, the birds were using their wings to stand. Both live and dead birds were submitted to the local field veterinarian for postmortem examination.
Gross Pathology: The veterinarian noted that the majority of the birds had pale streaking of the breast and thigh muscles and some birds also had pale streaking of the myocardium. Formalin-fixed tissues, frozen tissues, bacteriology swabs and a feed sample were submitted to the laboratory for further testing.
Laboratory results: PCR testing of lung/trachea was negative for Avian Influenza and Avian Paramxyovirus-1 infection.
Bacterial culture of swabs from bone marrow and peritoneum was negative for bacterial pathogens.
Feed sample HPLC Ionophore Analysis: 36 ug/g salinomycin was detected. Levels of monensin, narasin and lasalocid were < 1 ug/g.
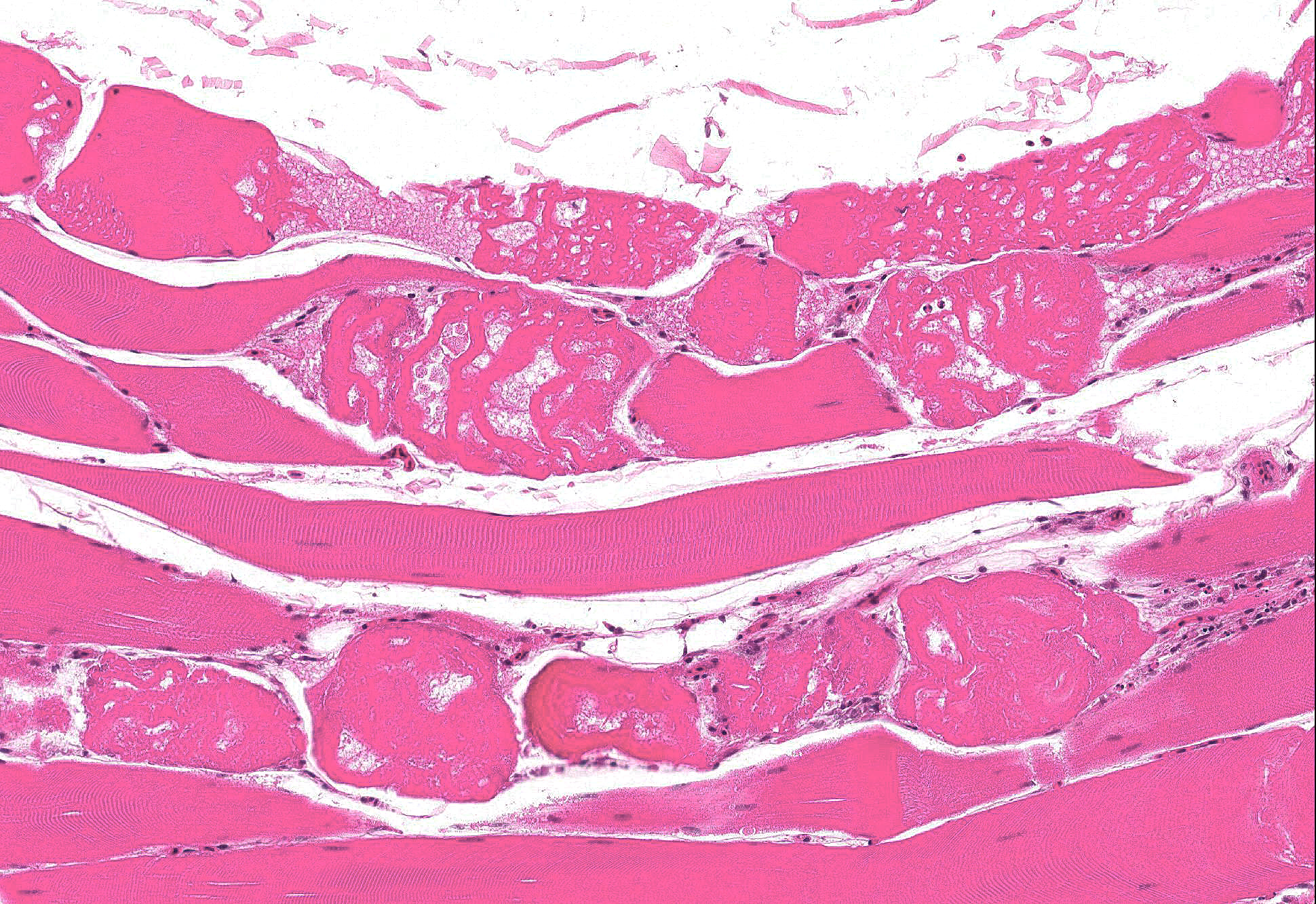
Microscopic Description: Skeletal muscle: Sections from the skeletal muscle from the thigh reveal widespread acute myonecrosis, with swelling, hyalinization, fragmentation and hypercontraction of myofibres. Numerous coagulated portions of myofibres are lightly mineralized and pale eosinophilic lightly fibrillar material is often present between the retracted necrotic myofibres. There is very early hypertrophy of satellite cell nuclei and low numbers of infiltrating macrophages and heterophils.
Contributors Morphologic Diagnosis:
Skeletal muscle: Acute severe multifocal monophasic skeletal myonecrosis.
Contributors Comment: Differential diagnoses for lesions of skeletal myopathy in turkeys include: exertional myopathy5, nutritional myopathy associated with Vitamin E/ selenium deficiency, and toxic myopathy caused by ingestion of ionophores or toxic plants such as Senna occidentalis3. Based on a strong index of suspicion for ionophore toxicosis, feed samples were submitted for testing, revealing 36 ppm of the ionophore salinomycin.
Several ionophores including monensin and lasalocid are approved for use in growing turkeys in Canada as an aid in the prevention of coccidiosis caused by Eimeria adenoides, E. meleagrimitis and E. gallopavonis. However, salinomycin, although approved for use in chicken broilers, is excluded from this list as it is known to be toxic to turkeys even at very low levels. A caution on the Canadian salinomycin label reads: Do not allow turkeys, dogs or horses access to this drug. It is known to be toxic to these species. Extra care should be taken to avoid contamination of feeds for these animals2. We have no further details as to how and when the salinomycin-contaminated feed was introduced to this specific flock of breeding turkeys, however, in most cases, there is a feed mill or on-farm feed mixing error, or salinomycin-medicated broiler feed is mistakenly fed to the turkeys.
Salinomycin can rapidly cause high mortality in affected flocks. In one recent case report, feed containing salinomycin was given to 13.5 weeks-old turkeys on May 29th. On June 2nd, the error was discovered and the salinomycin-medicated feed was removed but by June 3rd, more than 60% of the total mortality of 34.5% had already occurred6. The producer in the current case presented here lost almost all of the turkeys in this breeder flock as a result of ionophore toxicosis. Typical clinical signs include depression, stiffness, weakness, recumbency with extended legs, paralysis and death. The live turkeys in this case were described as panting and panting or dyspnea has been also been previously noted in turkeys with ionophore toxicosis1. Microscopic changes are reported in skeletal muscle and in some instances heart. Birds with respiratory signs often have lesions in tracheal muscles3, and these were noted on histology in this case, although lesions in the myocardium were minimal. Ionophores interfere with ion transfer at the cell membrane, facilitating movement of K+ ions out of myocytes and increasing Ca++ uptake. Toxicity is a result of skeletal muscle damage; type I fibers appear to be selectively affected3. Ionophore toxicity is dose dependent, species and age-dependent, and simultaneous use of other drugs may also augment toxicity in some cases1.
Evaluation of serum CK levels can be helpful in providing rapid confirmation of a skeletal myopathy when dealing with field cases of recumbency in turkeys, as differential diagnoses include botulism4.
JPC Diagnosis: Skeletal muscle: Degeneration and necrosis, multifocal, moderate, Meleagris gallopavo, avian.
Conference Comment: In domestic animals, toxic myopathies are generally caused by ingestion of ionophores, toxic plants, and plant-origin toxins. As a metabolically active organ system, skeletal muscle is highly susceptible to toxic injury resulting from membrane damage, altered protein synthesis, increased intracellular calcium concentration, or mitochondrial damage. Cases of myotoxicosis have variable clinical signs including: elevated serum concentrations of skeletal muscle cytoplasmic enzymes (CK and AST); severe muscle pain with or without myoglobinuria; or severe muscle weakness, recumbency and myoglobinuria. Animals often die from damage to cardiac muscle from the same toxin.3
Ionophores such as monensin, lasalocid, salinomycin, narasin, and maduramicin are compounds that alter membrane permeability to electrolytes by influencing transmembrane transport and function at low concentrations as a coccidiostat in birds and other animals. Monensin is the most common cause of ionophore toxicosis, and is produced by the fermentation of Streptomyces cinnamonensis. In addition to its function as a coccidiostat, monensin also promotes growth in ruminants. Toxicity results when animals are fed high concentration rations due to mixing errors, or when fed to monogastric animals that have a reduced tolerance to the drug; when medications are added to rations (tiamulin, triacetyloleandomycin, or sulfonamides) the toxic effects of ionophores are potentiated. Maduramicin is another ionophore antibiotic and a common coccidiostat in poultry that has demonstrated cardiotoxicity in cattle and sheep. With ionophore toxicity clinical signs can vary; however, with the administration of large single doses, birds may present with lethargy, stiffness, muscular weakness, and recumbency within 24-hours of ingestion. Toxic effects are cumulative with the ingestion of smaller doses, culminating in myocardial lesions and cardiac failure within 2-3 weeks. Myocardial lesions predominate in horses, in contrast to sheep and pigs where skeletal muscle damage with myoglobinuria is more prevalent. . In cattle, both skeletal and cardiac muscles appear equally affected. Microscopically, ionophore toxicity is characterized by multifocal monophasic necrosis of both types 1 and 2 muscle fibers with macrophage infiltration within 48 hours of ingestion; this differs from nutritional myopathies which cause polyphasic necrosis. Ultrastructurally, mitochondria are swollen and degenerate due to disruption of the membrane transport of sodium and potassium, leading to increased intracellular calcium and mitochondrial failure.3
Common plants that result in toxic myopathy are: Cassia occidentalis or C. obtusifolia (senna or coffee senna beans) and Karwinskia humboldtiana (coyotillo). Similar to ionophores, ingestion of these plants leads to weakness and eventual recumbency with skeletal muscle pallor, and microscopic multifocal monophasic myonecrosis.3
Gossypol is a polyphenolic substance found in cottonseeds (Gossypium spp.) that is toxic to most domestic animals (particularly, swine), causing lesions in several organs including the heart, skeletal muscle, liver, and lung. Similar to the aforementioned toxins, death is due to cardiac failure and monophasic myonecrosis; also, there is hepatic centrilobular necrosis and pulmonary edema.3
Additionally, the following plants have been known to cause toxic myopathy in select species: Diaporthe toxica (lupinosis in sheep), Cicuta douglasii (sheep with water hemlock), Thermopsis montana (calves with false lupine), Ageratina spp. (horses and ruminants with white snake root), Isocoma pluriflora (rayless goldenrod), Acer negundo (horses ingesting hypoglycin A found in the box elder tree), and selenium toxicosis in pigs, cattle, sheep and other domestic species.3
Unless there is known history of ingestion or the presence of the toxin in the intestinal tract, these toxic myopathies are impossible to differentiate.
Animal Health Laboratory
University of Guelph
Guelph, Ontario, Canada
References:
1. Andreasen JR, Schleifer JH. Salinomycin toxicosis in male breeder turkeys. Avian Dis.1995; 39 (3):638-642.
2. Bayley A. Bio-Cox 120 G. In: Inglis S, ed. Compendium of Veterinary Products. 11th ed. Hensall, ON: North American Compendiums, Ltd; 2009: 288.
3. Cooper BJ, Valentine BA. Muscle and tendon. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 1. 6th ed. Philadelphia, PA; Elsevier: 218-220.
4. Fulton RM. Other toxins and poisons. In: Saif YM, ed. Diseases of Poultry. 12th ed. Ames, IA: Blackwell Publishing; 2008:1231-1258.
5. Neufeld J. Salinomycin toxicosis of turkeys: serum chemistry as an aid to early diagnosis. Can Vet J.1992; 33: 677.
6. Williams S. Muscular system. In: Fletcher OJ, ed. Avian Histopathology. 3rd ed. Madison, WA: American Association of Avian Pathologists;2008:80-95.
7. VanAssen EJ: A case of salinomycin intoxication in turkeys. Can Vet J. 2006; 47 (3): 256-258.