WSC 2023-2024, Conference 12, Case 4
Signalment:
11-year-old spayed female Maine Coon cat (Felis catus)
History:
This animal had access to the external environment and exhibited a recognized propensity for predation. No other cats shared the same household. The cat presented with multiple indolent nodular skin lesions ranging from 5 to 20 mm in diameter around both eyes, on the right ear-base region, and on the ventral aspect of the tongue, as well as an ulcerated skin nodule on the tail. The cat was referred to a veterinary ophthalmologist. A cutaneous mycobacteriosis was diagnosed through cytological and histological examination. Surgical excision accompanied by a 4-week course of antimicrobial therapy (rifampicin combined with clarithromycin) was implemented. A culture followed by the molecular characterization of the isolate identified Mycobacterium lepraemurium in the resected periocular nodule.
The animal remained free from recurrences for at least one year following the surgical intervention; however, the cat subsequently experienced a relapse and was euthanized. The case was referred to the Institute of Veterinary Pathology of Zurich (IVPZ) for necropsy.
Gross Pathology:
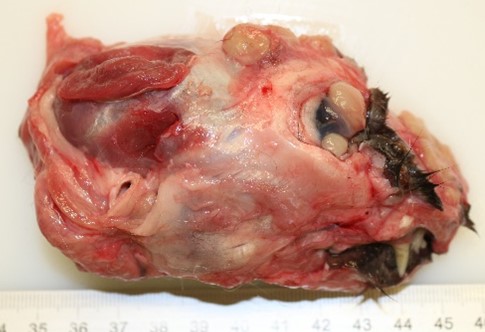
The skin and subcutis, particularly on the head, dorsal region, distal limb area, and the periocular region, conjunctiva, and cornea, presented numerous randomly distributed, well-circumscribed, alopecic, beige, soft, elevated nodules with sizes varying between 0.2 and 1.5 cm in diameter. The nodules in the rostral facial region were infiltrating and destroying the osseous structures (os nasale, os incisivum, nasal conchae).
Microscopic Description:
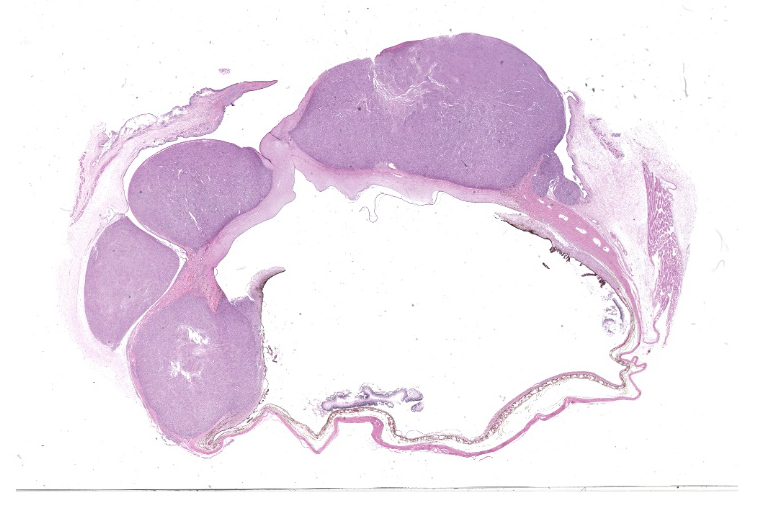
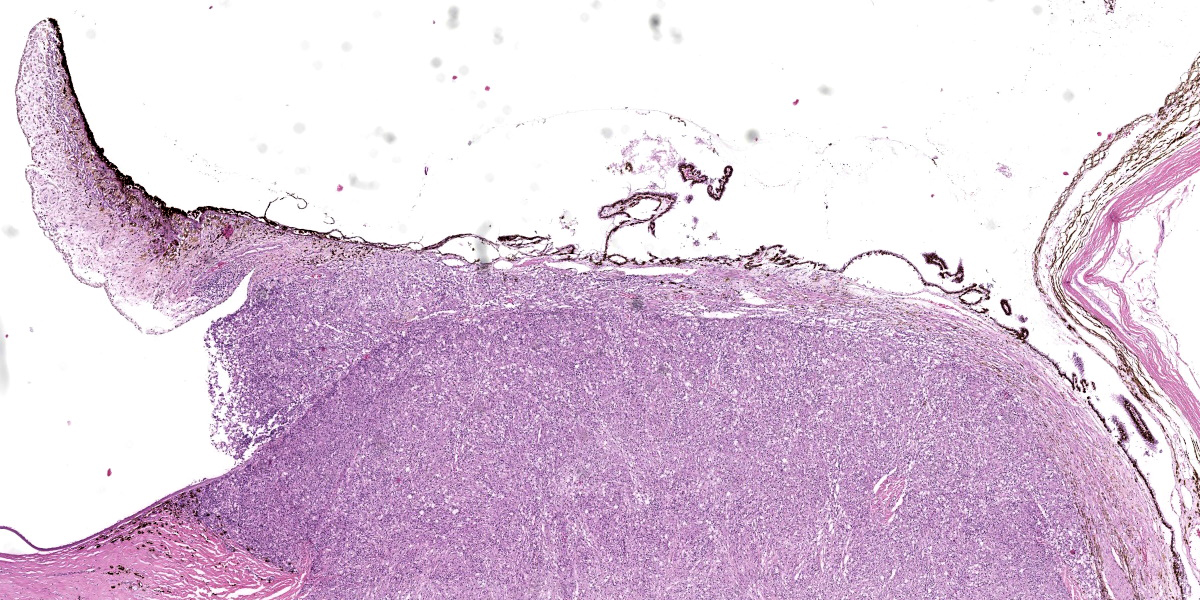
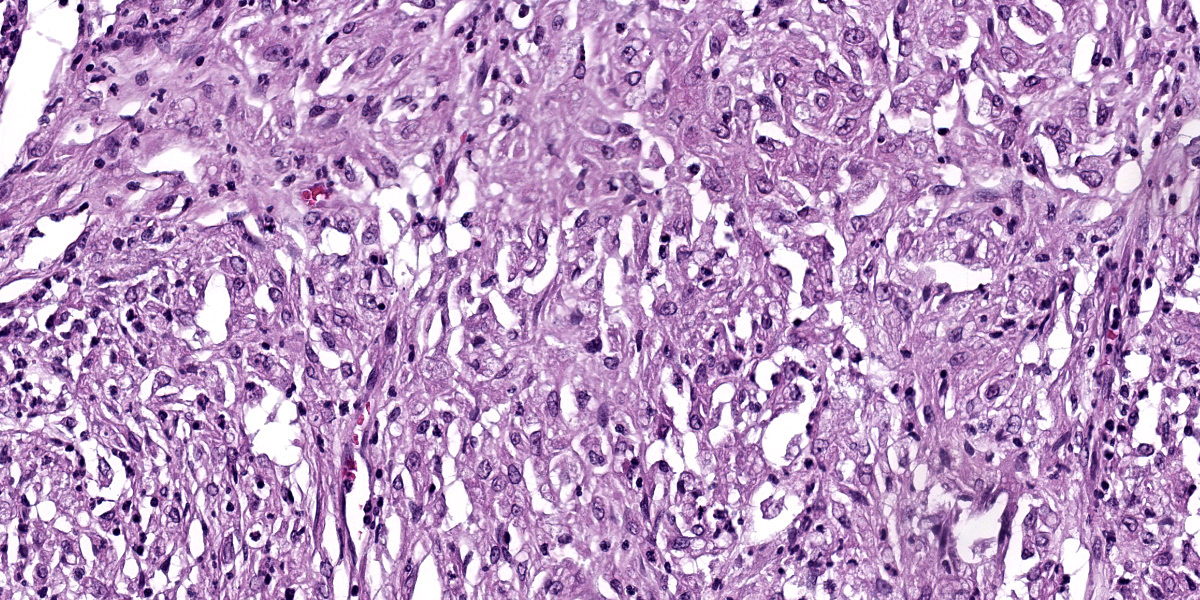
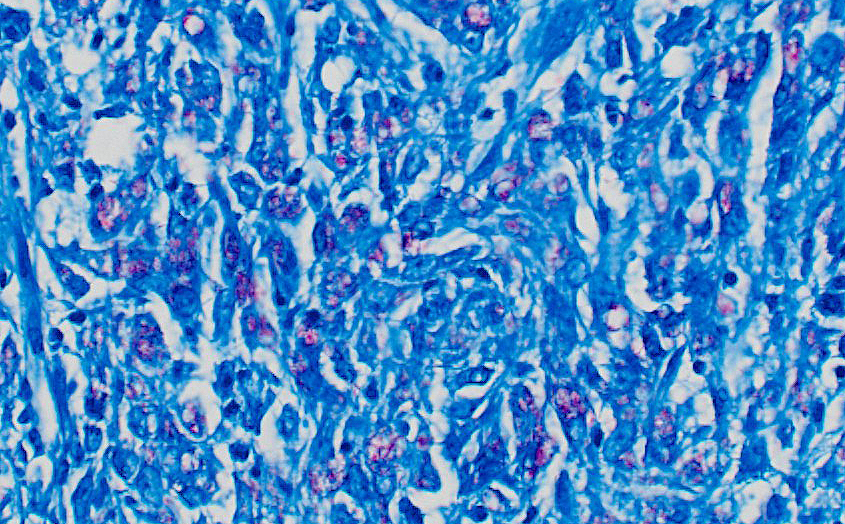
Right eye: Within the cornea and sclera, infiltrating and expanding the ciliary body and the iris and obliterating the irido-corneal angle with focal disintegration of Descemet’s membrane are multiple, well-circumscribed, coalescing granulomas ranging up to 1.2 cm in diameter. The granulomas contain numerous epithelioid macrophages, occasional multinucleate giant cells (foreign body type), scattered small lymphocytes, plasma cells, fibroblasts, fibrous connective tissue, and neutrophils. Occasionally, nodules contain a central area of necrosis characterized by abundant eosinophilic cellular and karyorrhectic debris. The cytoplasm of macrophages contains numerous negative staining rod-shaped structures. The corneal epithelium, which lines the distended corneal stroma affected by granulomas, exhibits multifocal areas of ulcerations characterized by loss of epithelial lining cells. Numerous smaller granulomas are present interspersed among the connective tissue of the eyelid and conjunctiva.
A Ziehl-Neelsen acid fast stain revealed myriad intrahistiocytic and free, 0.5 x 2 µm, acid-fast filamentous bacilli.
Contributor’s Morphologic Diagnosis:
Globe: Severe, multifocal to coalescing, chronic granulomatous keratoconjunctivitis and anterior uveitis with numerous intrahistiocytic and extracellular acid-fast bacilli.
Contributor’s Comment:
Mycobacteria in cats can have different clinical presentations, ranging from localized cutaneous lesions to generalized, systemic infections. Mycobacteria are categorized into three main groups.
The Mycobacterium tuberculosis complex (MTBC) contains 9 closely-related host adapted species with varying ability to cross species barriers. Among cats, the most prevalent species from this group (in the UK) are the rodent-adapted Mycobacterium microti and the cattle-adapted Mycobacterium bovis.6 The lesions caused by MTBC are usually present in skin and lymph nodes as an effect of bite and fighting wounds, but may also be present in the lungs, gastrointestinal tract, and rarely, joints.8
Non-tuberculosis mycobacteria (NTM) include mainly opportunistic organisms that may be found in the environment, including water, soil, aerosols, and vegetation. In cats,
the species of main concern are Mycobacterium malmoense, Mycobacterium branderi/ shimoidei, and Mycobacterium avium. The infections caused by pathogens from this group are usually systemic and have a high shedding potential given the fact that extracellular bacilli are found in respiratory and intestinal epithelium as well as in glomerular tufts.7
The Mycobacterium leprae complex (MLC) causes “feline leprosy syndrome” (FLS) and includes M. lepraemurium, M. visibile, and Mycobacterium sp. strain Tarwin. The standard microbiological methods are not effective for the culture of these species. Clinical presentation of the infection is usually limited to skin, and the dermal lesions are nodular, often ulcerated, and present predominantly on the head and the forelimbs. The generalized nodular skin disease consists of numerous disseminated skin nodules and is a rarely observed pattern of infection. Despite the predisposition of M. lepraemurium to cause damage in the head area, it does not tend to involve the eyes. In contrast, Mycobacterium sp. strain Tarwin can cause feline leprosy with a particular propensity to produce lesions on the head, particularly involving the eyes and periocular skin.5
Histologically, the lesions seen in cats affected by feline leprosy syndrome can be divided into two categories. Lepromatous leprosy is thought to be the malignant form and is accompanied by numerous, typically intracellular acid-fast bacilli (multibacillary), whereas the tuberculoid leprosy is frequently recognized as the benign form of the disease and is defined by the presence of relatively small numbers of intrahistiocytic acid-fast bacilli (paucibacillary).2
The most likely method of transmission for M. lepraemurium, which causes leprosy in cats and rodents, is through the bites of infected prey during hunting. It has not yet been established whether there is a niche in the environment where the pathogen can survive without its host; however, the clinical and histological findings in wild rodents, where the pathogen is well adapted, and cats serving as incidental hosts seem to support this hypothesis.1
Contributing Institution:
Institut für Veterinärpathologie
Vetsuisse-Fakultät, Universität Zürich
https://www.vetpathology.uzh.ch
JPC Diagnosis:
Eye: Keratoconjunctivitis, scleritis, and uveitis, pyogranulomatous, multifocal to coalescing, severe.
JPC Comment:
As the contributor notes, Mycobacterium lepraemurium does not typically involve the ocular tissues; in fact, this is the only case of ocular mycobacteriosis due to M. lepraemurium reported in Europe to date.1,3 In a recent study of ocular mycobacteriosis in cats, tuberculosis (infection with Mycobacterium bovis, Mycobacterium microti, or a nonspeciated Mycobacterium tuberculosis complex pathogen) was diagnosed in 91% of cats, while Mycobacterium lepraemurium was identified in only 9% of cats, providing further evidence for the relative rarity of this pathogen in ocular tissues.3 The study found that the choroid, retina, ciliary body, and sclera were the tissues most affected in ocular disease caused by mycobacterial organisms other than M. lepraemurium, with inflammation being most florid within the choroid.3 By contrast, the two ocular cases of M. lepraemurium infection contained lesions restricted to the cornea, sclera, and conjunctiva.3 Infection restricted to the external tunic of the eye is typical of this infection and may be due to the inability of M. lepraemurium to replicate well at higher body temperatures.3
The division of feline leprosy syndrome into lepromatous or tuberculoid forms is based on the differential elaboration of canonical cytokines by CD4+ T lymphocytes. In the tuberculoid form, Th1 lymphocytes produce large amounts of IL-2 and interferon gamma, resulting in a cell-mediated immune response rich in activated macrophages and T-cells which, in a virtuous immune feedback loop, stimulate mutual proliferation and activation and the subsequent containment of infection.4 Lesions of this type are paucibacillary due to the low numbers of surviving infectious organisms.
By contrast, lepromatous disease produces multibacillary lesions that are driven by a Th2 lymphocyte response. In these cases, the Th2 lymphocytes produce a cytokine milieu rich in IL-4, IL-10, and IL-13, all of which suppress macrophages and stimulate B lymphocytes, and IL-5, which recruits eosinophils. The resulting humoral response stifles cell-mediated immunity and allows infections to progress, resulting in more diffuse, multibacillary infections such as that present in this case.4 In vivo, these responses do not exist as discrete entities, but rather occur on a continuum with the relative contribution of Th1 and Th2 responses driving disease progression and clinical outcomes. In fact, the recent study discussed above found that high bacterial loads were found with infections with M. bovis, M. microti and M. lepraemurium, indicating that the presence of multibacillary lesions is not necessarily restricted to infections with non-tuberculosis mycobacteria and Mycobacterium leprae complex pathogens.4
Conference participants discussed the origin of these stiking lesions and debated whether the infection was of hematogenous or external, “outside-in” origin. Given the gross appearance and the nodules arising from the conjunctiva and cornea, participants decided that an external origin was most likely. Participants also discussed whether the discontinuity in Descemet’s membrane was real or artifactual, before deciding that the lesion is real due to the highly reactive underlying stroma. Elsewhere in the cornea, some loss of stromal clefting was noted, but Dr. Neto cautioned resident against overinterpreting corneal edema in a necropsied animal as some post-mortem corneal aqueous absorption is common.
Dr. Neto also discussed the choice of carbol-fuchsin stains for acid-fast organisms. In general, she finds Ziehl-Neelsen to be less effective for non-tuberculous Mycobacterium as the solvent used may rapidly remove dye from the organisms’ thinner walls; however, Fite-Faraco has can cause indiscriminate staining of necrotic material, making Ziehl-Neelsen the stain of choice in the face of abundant necrosis.
References:
- Ghielmetti G, Schmitt S, Friedel U, Guscetti F, Walser-Reinhardt L. Unusual presentation of feline leprosy caused by Mycobacterium lepraemurium in the alpine region. Pathogens. 2021;10(6):687.
- Juarez-Ortega M, Hernandez, VG., Arce-Paredes P, Villanueva EB, Aguilar-Santelises M, Rojas-Espinosa O. Induction and treatment of anergy in murine leprosy. Int. J. Exp. Pathol. 2014;96:31-41.
- Mitchell JL, MacDougall L, Dobromylskj MJ, et al. Ocular mycobacterial lesions in cats. Vet Pathol. 2022; 59(5):792-805.
- Modlin RL. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol. 1994;102(6):828-832.
O’Brien CR, Malik R, Globan M, Reppas G, McCowan C, Fyfe JA. Feline leprosy due to Candidatus ‘Mycobacterium tarwinense’: Further clinical and molecular