WSC 2023-2024, Conference 23, Case 2
Signalment:
Juvenile female sandhill crane (Antigone canadensis).
History:
The animal was presented to a local wildlife center with a cup attached to its beak. On initial exam, the submitting veterinarian noted emaciation, dehydration, and indentation on the beak as well as swelling of the right hock and left tibiotarsus, with no obvious abnormalities on radiographs. The bird was moved to an outside rehabilitation space 1 day after presentation and was eating and gaining weight reliably. The animal was found deceased a few weeks later.
Gross Pathology:
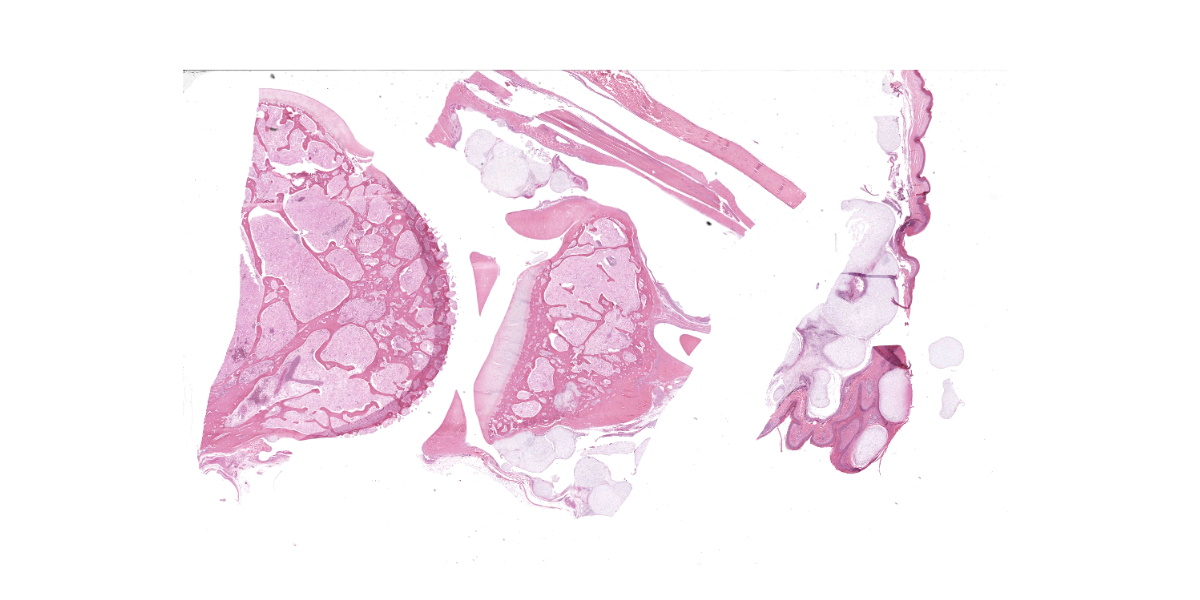
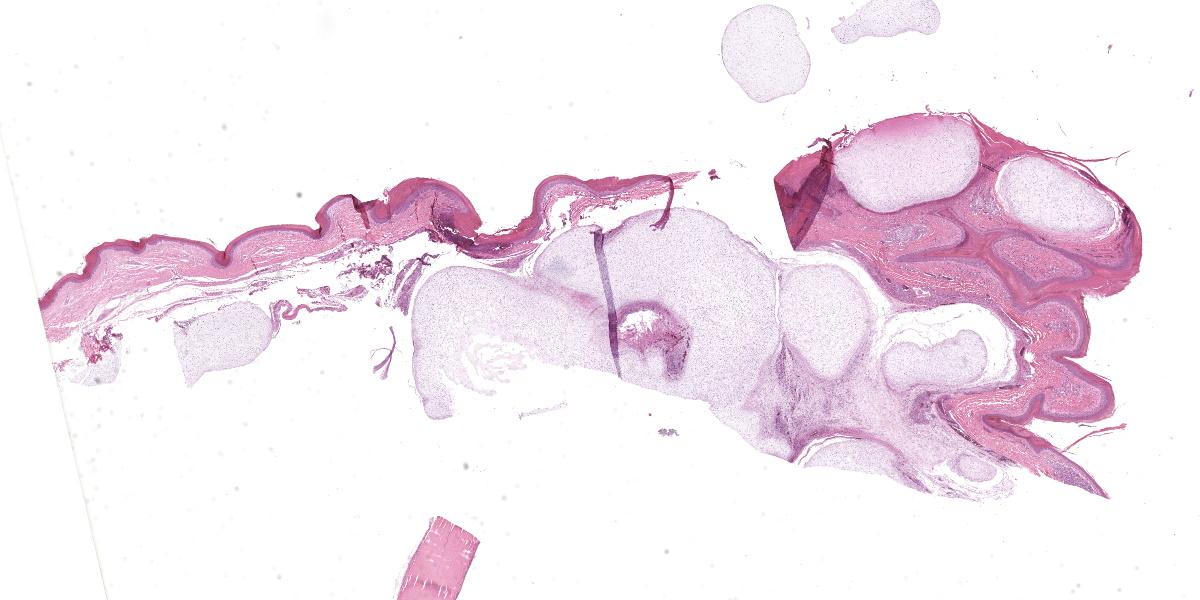
The animal weighs 2.2 kg and is in thin physical condition as evidenced by atrophied pectoral musculature and adipose tissue. There is a concentric deep indentation midway along the length of the beak. Overlying the right ankle are multiple firm, occasionally ulcerated nodules that are 2 to 5 mm in diameter. When sectioned the nodules are pale blue or tan, firm, and translucent with a botryoid appearance. Dissection of the joint shows many similar nodules firmly attached to the dermis, soft tissues, ligaments, and tendons. The condyle of the tibiotarsus has a fracture with pitting and marked proliferation of white firm nodules in the defect and epicondyle.
There is a focal, firm, nodular swelling overlying the distal left tibiotarsus that measures 1.8 cm x 0.8 cm. When sectioned, the nodule is tan and translucent. Relative to the right pectoral muscle, there is moderate wasting of the left pectoral musculature.
Within the mid-trachea at the level of the reflexive curvature are multiple intraluminal trematodes. The organisms are 1.4 cm long, flat and fusiform with a bulbous anterior end; they are white with bilateral dark brown linear striations. Within the affected area, the tracheal mucosa is red and slightly thickened.
There are no significant gross abnormalities in the tongue, esophagus, lungs, heart, liver, spleen, pancreas, kidneys, proventriculus, ventriculus, small intestine, large intestine, or brain. The spinal cord is not examined in this case.
Microscopic Description:
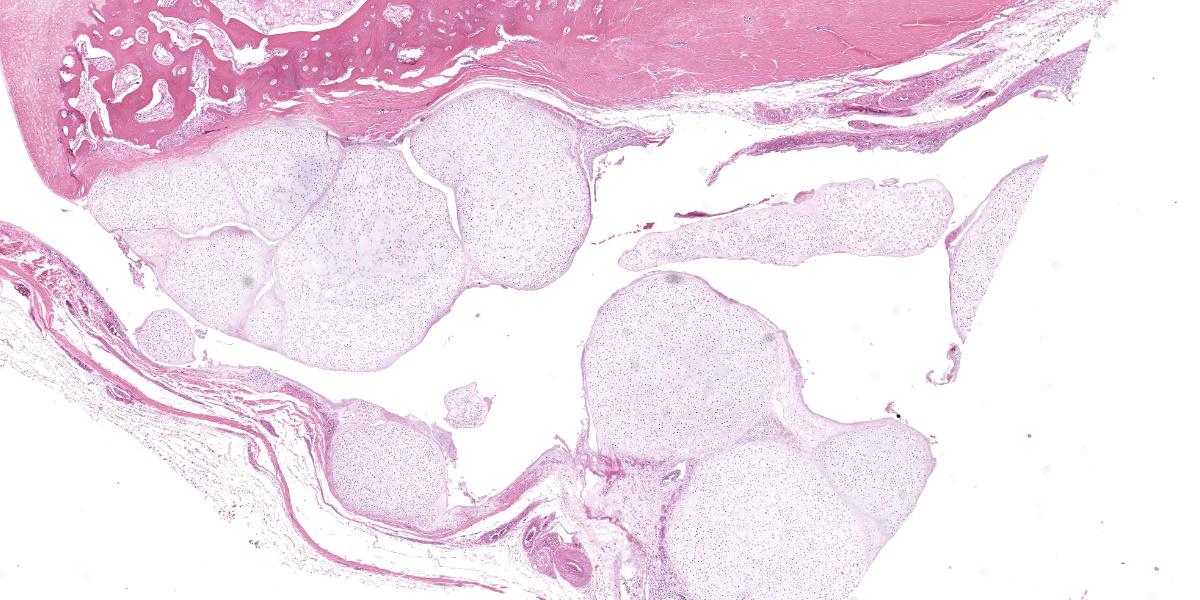
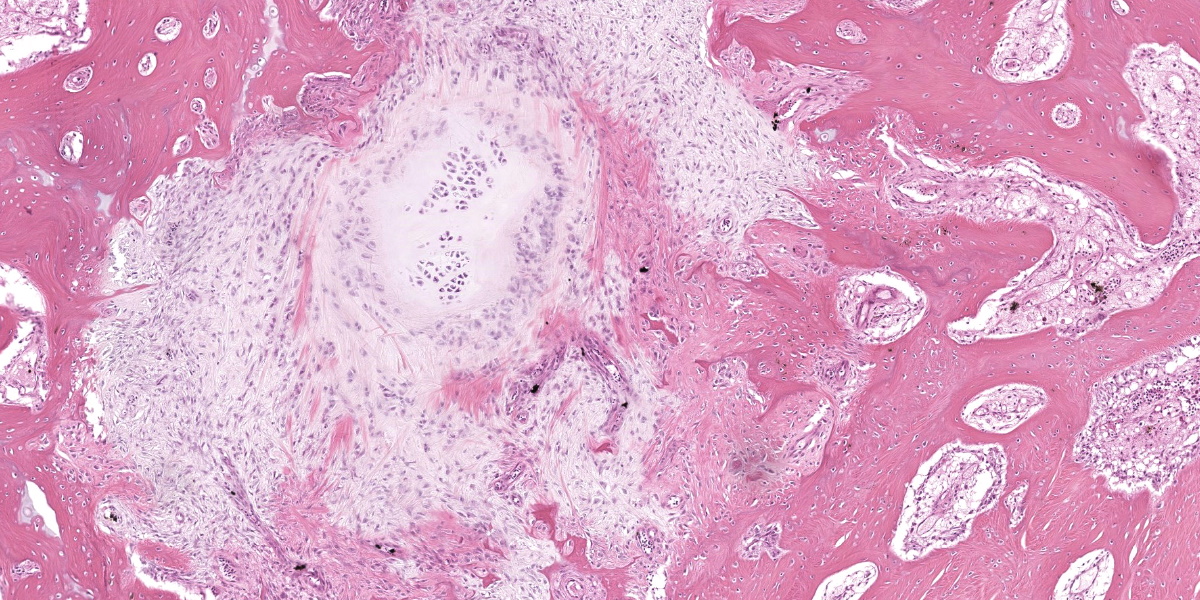
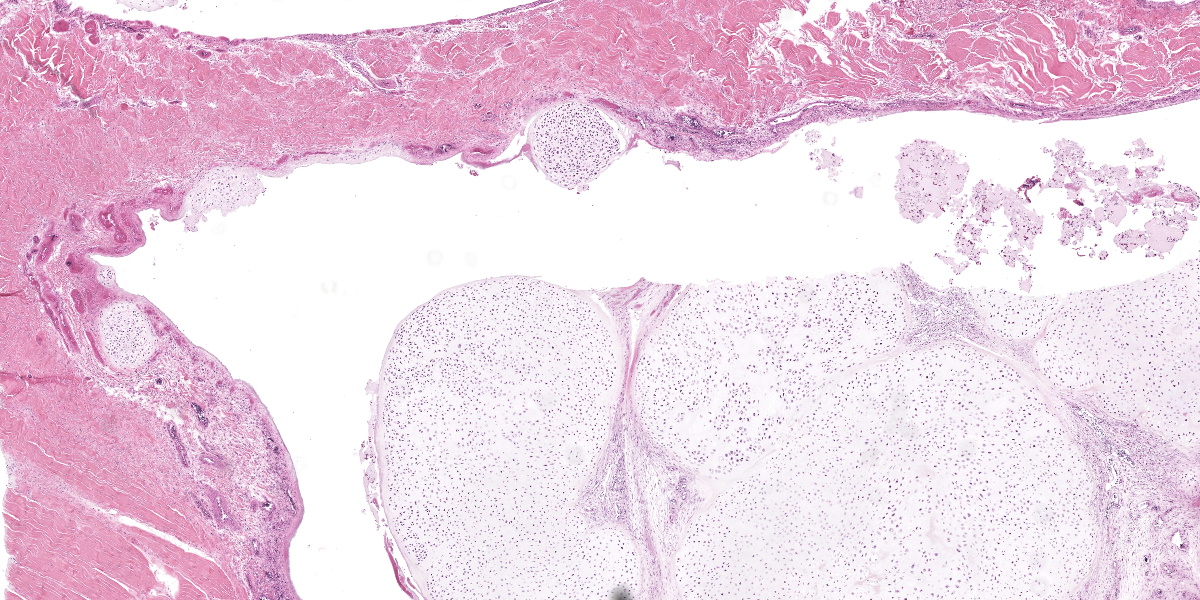
Ankle joint: The bone profile is irregular due to many cartilage nodules that arise from the synovium of the joint spaces (synovium) and tendon sheaths (tenosynovium). Nodules contain many evenly spaced chondrocytes in hyaline pale blue matrix; they have moderate finely vacuolated pale purple cytoplasm and large round nuclei with peripheralized chromatin and 1-3 nucleoli. Anisocytosis and anisokaryosis are marked. Mitoses are not evident. Binucleate cells are occasionally evident, and a trinucleate cell was seen. Many of the chondrocytes are pyknotic or karyolytic with pink cytoplasm (necrosis); such cells are dispersed between viable cells or predominate in the center of nodules where there is fragmentation of matrix and rarefaction of tissue. In larger nodules, the central chondrocytes cluster with intervening areas of acellular matrix. The synovium is thin and often necrotic when stretched over the nodules, with small thrombi in the associated vessels. In other areas the subintima is thickened by large mesenchymal cells embedded in mucinous matrix and variable numbers of lymphocytes, plasma cells, and heterophils. Scant fibrin and karyorrhectic cells are present in the joint spaces. The bone surfaces have mild remodeling, there is marked serous atrophy of marrow fat, and skeletal myocytes are atrophied. The skin bulges and is ulcerated due to several cartilage nodules in the subcutis, dermis, and epidermis. Synovium surrounds the intradermal nodules and merges with the inflamed and fibrotic dermis. Intraepidermal nodules are demarcated from the dermis by many heterophils; their cartilage matrix is pale blue to pink, and cells are pyknotic along the subcorneal surface. The epidermis overlying the intraepidermal nodules is necrotic and the surrounding epidermis is hyperplastic (acanthosis, increased mitoses). The stroma around and deep to the intraepidermal nodules is expanded by many red cells, heterophils, and compact collagen that has many fine karyorrhectic fragments, as well as small thrombosed vessels.
Contributor’s Morphologic Diagnoses:
- Tibiotarsus: Moderate chronic heterophilic synovitis with synovial and tenosynovial chondromatosis.
2. Skin: Multifocal chondromatosis with epidermal hyperplasia and necrosis, heterophilic dermatitis, and transepithelial elimination
Contributor’s Comment:
Synovial chondromatosis (also known as synovial chondrometaplasia) is an uncommon non-neoplastic process characterized by the formation of multiple cartilage nodules in synovial tissues of a joint, tendon sheath, or bursa. When the majority of these nodules undergo endochondral ossification, the term osteochondromatosis is used.2,3,7 Reports in animals have mostly been in dogs1,7,8,10,14 and raptors11,15,18 but horses and pigs can also be affected.6,13 It should be noted that osteochondromatosis of cats is different in its biological behavior, distribution, and possible etiology, and thus should not be combined when discussing the disease entity in other species.3
Males predominate in reports from both dogs and humans, while the few reports in raptors have increased numbers of females.3,11,15,18 In dogs most reports are from large and giant purebreds from 7 months to 11 years old. The shoulder joint is most often affected, although cases have also been observed in the stifle, elbow, hock, and digits.2,3 Clinical signs include chronic progressive lameness with swelling of the joint.1,7,8,10
In raptors, the majority of cases are reported bilaterally in the scapulohumeral joints of great horned owls (Bubo virginianus), with or without osteoarthritic change. Fewer cases of chondromatosis in the stifle joints have been reported.11,15,18 Birds often present in poor nutritional condition due to severe mobility restriction. This case is the first report of chondromatosis in the Gruiformes group and affecting the ankle joints.
Two forms of synovial chondromatosis (primary and secondary) have been described. Primary (idiopathic) chondromatosis occurs spontaneously within the synovium of a single, otherwise unremarkable joint; it is rare in humans and animals. Grossly, primary synovial chondromatosis presents with more nodules than secondary chondromatosis.3 The pathogenesis of primary synovial chondromatosis is unknown, and it is thought to be an idiopathic nodular cartilaginous metaplasia of the synovium. Secondary chondromatosis is thought to be a reaction to a traumatic, degenerative, or inflammatory joint disease. Speculatively, the etiology in raptors is thought to be a chronic inflammatory process due to joint strain.15 In older horses and dogs, it is usually an incidental finding at necropsy, while in raptors it is often associated with the demise of the individual due to an inability to fly.3,15 Nodules are reported to be less numerous and mixed with other forms of synovial proliferation and metaplasia. Erosive changes in the articular cartilage are often more marked than the degree of synovial proliferation.3 In this case, the bilateral occurrence in the ankles, presence of synovitis, and fracture of the tibiotarsus indicates secondary synovial chondromatosis.
Classically, gross lesions are described as multiple firm or hard white nodules embedded in the synovium and periarticular connective tissues, with variably intact articular cartilage.2,3,14 The nodules consist of islands of highly cellular hyaline cartilage covered by a layer of synoviocytes or fibrous tissue attached to or continuous with the synovial lining of joints or bursae. Chondrocytes may be haphazardly clustered, large, and sometimes binucleate. In this case, trinucleate chondrocytes were rarely evident. Areas of fibrocartilage, central necrosis, or endochondral ossification with medullary hematopoiesis may also be present. In some cases proliferative plasmacytic synovitis is reported.3,17 Nodules may detach from the synovium and float freely in the joint. In these cases, they are termed “loose bodies” and may undergo ischemic necrosis.2,12 Extra-articular chondromatosis has been rarely reported; in this case chondrometaplasia was evident in a tendon sheath.7
Under certain circumstances, nondermal elements enter the epidermis and are eliminated to the outside, a process termed ‘transepithelial elimination’ (TE).12 Four subdivisions of TE are described, two of which involve large portions of tissue, as in our case (subdivisions 3 and 4). As migrating tissues compress the epidermis from below, epithelial damage occurs with necrosis and ulceration, and possible sloughing.12 Transepithelial elimination, to the authors’ knowledge, has not been reported in association with synovial chondromatosis or in the class Aves. In this case, TE probably occurs as a result of the pressures exerted on the nodules by the relatively immobile and non-distensible skin of the ankle in birds. This area may also have increased susceptibility to ulceration versus a more protected scapulohumeral lesion, which is more often seen in birds with chondromatosis.
Differential diagnoses for synovial chondromatosis include chondroma and chondrosarcoma. Chondroma is a benign neoplasm of cartilage that has been occasionally reported in multiple veterinary species. Histologically, chondromas may have similar irregular lobules of hyaline cartilage, which may also have foci of endochondral ossification and mineralization. In fact, some report that primary chondromatosis may be a multifocal form of a chondroma.5,6 An important differential diagnosis is synovial chondrosarcoma. Malignant transformation of synovial chondromatosis to chondrosarcoma is rarely reported in dogs.1,7 Features that suggest chondrosarcoma are bone invasion and replacement of the chondrocyte cluster pattern with a more random distribution of cells.1,3,7
Contributing Institution:
Veterinary Diagnostic Laboratory
University of Illinois at Urbana-Champaign
Urbana, IL
https://vdl.vetmed.illinois.edu/
JPC Diagnosis:
Joint capsule, tendon sheath, and overlying skin: Chondromatosis, nodular, multifocal, severe, with intra-osseous extension, transepidermal elimination, and chronic tenosynovitis and dermatitis.
JPC Comment:
The contributor provides an excellent summary of synovial chondromatosis (SC) as this photogenic entity presents in dogs, birds, pigs, and horses. Cats, however, long known for their flair for the idiosyncratic, have a similar sounding but distinct condition, feline osteochondromatosis, which is distinct from the synovial chondromatosis discussed above and from osteochondromatosis in other animals.16 In horses, dogs, pigs, and humans, osteochondromatosis demonstrates an autosomal dominant inheritance pattern and typically occurs in young animals only during the period of active bone growth.16 The cartilage-capped tumors that arise from bone surfaces give the condition its name and tumor growth arrests once growth plates close.16
In contrast, feline osteochondromatosis usually presents in adult cats (most commonly 2-4 years old) long after skeletal maturity is achieved. The condition presents with no breed or sex predisposition and does not appear to be inherited. The disease affects the axial skeleton and, in a sad departure from the tumor arrest seen in other animals, tumors show progressive growth and can undergo malignant transformation to osteosarcoma or chondrosarcoma.16
Interestingly, feline osteochondromatosis is usually diagnosed in cats concurrently infected with feline leukemia virus (FeLV), leading to speculation that FeLV infected fibroblasts account for the special pathogenesis and distinct clinical behavior of osteochondromatosis in cats relative to other species.9,16 FeLV particles have been visualized within feline osteochondromatous tissue via electron microscopy and via in situ hybridization; however, a direct pathogenetic link between FeLV infection and the condition has not been conclusively established, and osteochondromatosis has been reported in FeLV-negative cats.9,16
In humans, dogs, and a few cats, osteochondromatosis has been associated with premature stop codon-producing frameshift mutations in the exostosin glycosyltransferase 1 and 2 genes (EXT1 and EXT2, respectively), whose protein products form an enzyme complex that modifies heparan sulfate chains.4 It is thought that heparan sulfate chain dysfunction can scramble chondrocyte differentiation or proliferation pathways, fibroblastic growth factors, bone morphogenic proteins, and Wnt signalling pathways, all of which could account for the lesions of osteochondromatosis.4 These theoretical pathogeneses have not been proven in human or in veterinary medicine, and in all species tested, EXT1 and EXT2 mutations are absent in an significant subset of osteochonromatosis cases.4
One such recent case report detailed the radiographic and pathologic findings of feline osteochondromatosis in an aged, 12-year-old Russian Blue, FeLV-negative cat with a years-long history of progressive forelimb lameness.9 Radiographs revealed bilateral, calcified proliferative lesions of the elbow joints and multiple vertebral bodies and ribs. Grossly, the elbow joints were completely ankylosed by large, round masses containing white material that replaced normal tissue architecture; the affected vertebrae and ribs contained similar lesions.9 Testing for EXT1 and EXT2 mutations and FeLV infection were all negative, confirming that this condition can arise spontaneously in aged cats.9
Back in the joints of the sandhill crane, the moderator noted that this, along with vertebral angiomatosis, was yet another entity with bland looking cells that nevertheless have no business being where they are. Conference participants noted the lack of anisocytosis, anisokaryosis, and mitotic figures, all of which point toward a benign process; however, the intra-osseous nodule of cartilage, which should not be present in a pure case of synovial chondromatosis, raised the spectre of chondrosarcoma for some participants. Participants ultimately decided that the bland histologic appearance of the cells likely a precluded chondromsarcoma diagnosis, but the phrase “with intra-osseous extension” was added to the morphologic diagnosis to capture this unusual feature.
References:
- Aeffner F, Weeren R, Morrison S, Grundmann INM, Weisbrode SE. Synovial osteochondromatosis with malignant transformation to chondrosarcoma in a dog. Vet Pathol. 2012;49(6):1036-1039.
- Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 1. 2016;Elsevier:161-163.
- Craig LE, Thompson KG. Tumors of Joints. In: Meuten DJ, ed. Tumors in domestic animals. 5th ed. John Wiley & Sons Inc;2016:337-355.
- D’Arienzo A, Andreani L, Sacchetti F, Colangeli S, Capanna R. Hereditary multiple exostoses: current insights. Ortho Res Rev. 2019;11:199-211.
- Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
- de Brot S, Grau-Roma L, Vidal E, Segalés J. Occurrence of osteochondromatosis (multiple cartilaginous exostoses) in a domestic pig (Sus scrofa domesticus). J Vet Diagn Invest. 2013;25(5):599-602.
- Díaz-Bertrana C, Durall I, Rial JM, Franch J, Fontecha P, Ramis A. Extra- and intra-articular synovial chondromatosis and malignant transformation to chondrosarcoma. Vet Comp Orthop Traumatol. 2010;23(4): 277-283.
- Edinger DT, Manley PA. Arthrodesis of the shoulder for synovial osteochondromatosis. J Small Anim Pract. 1998;39(8): 397-400.
- Gomez A, Rodriguez-Largo A, Perez E, et al. Feline osteochondromatosis in a 12-year-old feline leukaemia virus-negative cat. J Comp Pathol. 2003;25:24-26.
- Gregory SP, Pearson GR. Synovial osteochondromatosis in a labrador retriever bitch. J Small Anim Pract. 1990;31(11): 580-583.
- Howard MO, Nieves MA, Miles KG. Synovial chondromatosis in a great horned owl (Bubo virginianus). J Wildl Dis. 1996;32(2):370-372.
- Mehregan AH, Hashimoto, K. General Pathology: Terminology. In Pinkus’ Guide to Dermatohistopathology Appleton & Lan-ge;1995:83-100.
- Smith RK, Coumbe A, Schramme MC. Bilateral synovial chondromatosis of the metatarsophalangeal joints in a pony. Equine Vet J. 1995;27(3):234-238.
- Smith TJ, Baltzer WI, Löhr C, Stieger-Vanegas SM. Primary synovial osteochondromatosis of the stifle in an English Mastiff. Vet Comp Orthop Traumatol. 2012;25(2):160-166.
- Stone EG, Walser MM, Redig PT, Rings B, Howard DJ. Synovial chondromatosis in raptors. J Wildl Dis. 1999;35(1):137-140.
- Szilasi A, Koltai Z, Denes L, Balka G, Mandoki M. In situ hybridization of feline leukemia virus in a case of osteochondromatosis. Vet Sci. 2022;9(2):59.
- Villacin AB, Brigham LN, Bullough PG. Primary and secondary synovial chondrometaplasia: histopathologic and clinicoradiologic differences. Hum Pathol. 1979;10(4):439-451.
- Xie S, Nevis J, Lezmi S. Pathology in practice. Chondro-osseous metaplasia consistent with synovial chondromatosis in a great horned owl. J Am Vet Med Assoc. 2014;245(7):767-769.