Signalment:
Gross Description:
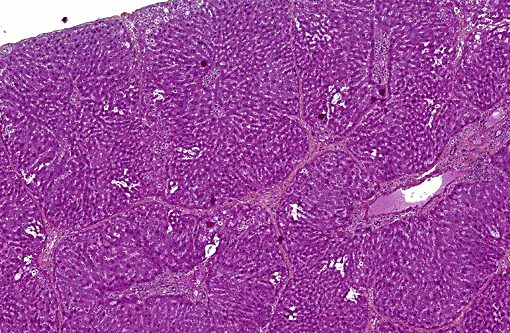
Histopathologic Description:
Morphologic Diagnosis:
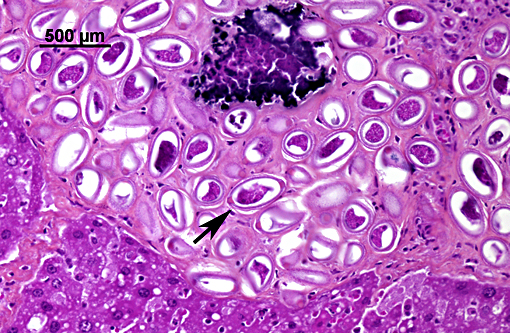
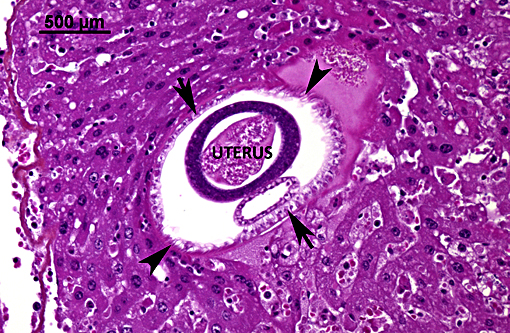
Liver: Hepatitis, granulomatous and eosinophilic, chronic, multifocal, moderate, with intralesional eggs and adult nematodes consistent with Capillaria hepatica (adults are not present on all slides).
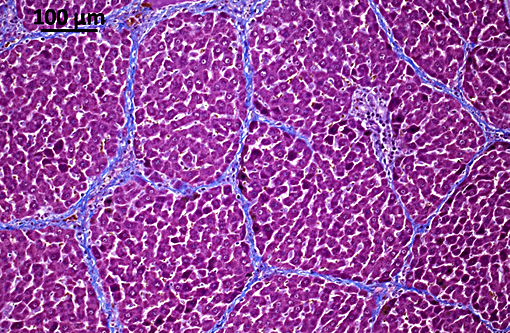
Liver: Porto-portal and porto-central bridging fibrosis, multifocal, moderate to severe (septal fibrosis).
Condition:
Contributor Comment:
Capillaria hepatica is the only known nematode with a direct life cycle requiring death of the host to be completed. Following their ingestion, embryonated eggs hatch in the intestine and release first stage larvae that cross the cecal barrier and reach the liver through mesenteric and portal veins. The migration of larvae within the hepatic parenchyma produces areas of hepatic necrosis.(10,11) After three weeks, mature females start to lay eggs around portal tracts until they die (up to seventy days later). After their death, adults progressively disintegrate. Eggs elicit a mixed inflammatory response composed of macrophages, multinucleated giant cells, lymphocytes, plasma cells and eosinophils that leads to the formation of granulomas.(10,11)
The peculiarity of hepatic capillariasis is that eggs are kept within the hepatic parenchyma instead of being released through the biliary tract as for other hepatic parasites. Eggs can only be released in the environment after death and decomposition of the host or after its ingestion by a predator. This predator represents a paratenic host that, although not necessary for completing the cycle, greatly promotes the maturation and the dissemination of the eggs.(10,11)
A peculiar finding in rats infected with Capillaria hepatica is the development of septal fibrosis, a type of bridging fibrosis in which portal tracts are connected to portal tracts (or rarely to centrilobular spaces) by thin strands of connective tissue containing collagen, fibroblasts and lymphocytes. This pattern gives the hepatic parenchyma a porcine liver-like architecture. In humans, septal fibrosis is an important, frequent and non-specific finding in chronic liver diseases. For this reason, rats infected with Capillaria hepatica represent a good model for hepatic fibrosis. It has been shown that in this model, septal fibrosis is not preceded by hepatic necrosis or overt chronic inflammation. The first step of septal fibrosis appears to be star-shaped expansions of portal spaces containing fibroblasts and blood vessels that sprout from periportal spaces. The presence of proliferating blood vessels is a characteristic feature.(2,3,5,7)
In the present case, other rodent species (ex: Greater white-toothed shrew (Crocidura russula)) have been trapped and revealed to be infected by Capillaria hepatica but only rats developed septal fibrosis. For veterinary pathologists, this example illustrates how different can be the patterns of tissue reaction among species, even with the same initiating cause. Another well-known example for that is tuberculosis.(1)
Table 1. Hepatic parasites in Domestic Species
| PARASITE | DEFINITIVE (DH) OR INTERMEDIATE (IH) HOSTS | LOCATION/COMMENT |
| WANDERING LARVAE (NUMEROUS SPECIES) | ||
| Cysticercus tenuicollis (Taenia hydatigena) | IH: sheep, goat, cattle, squirrel, swine | Cysts in peritoneal cavity / Fatal hepatic hemorrhages in lambs severely infected. Associated with Black disease |
| Cysticercus pisiformis (Taenia pisiformis) | IH: rabbit, squirrel, small rodents | Cysts in liver capsule |
| Cysticercus fasciolaris (Taenia teaniaeformis) | IH: rodents, rabbit | Cysts in liver / Associated with the development of fibrosarcoma |
| Larvae of Ascaris suum | DH: swine | Causes milk-spotted liver. Adults in intestine |
| Larvae of Stephanurus dentatus | DH: swine | Adults in urinary system |
| Larval strongyles (Strongylus edentatus,S. equi, S. vulgaris) | DH: horse | Causes villous perihepatitis |
| CESTODES | ||
| Stilesia hepatica | DH: ruminants (Africa) | Only cestodes that inhabit the bile ducts |
| Thysanosoma actinioides | DH: ruminants (America) | |
| Echinococcus granulosus | IH: humans, cattle, swine, sheep, deer, horse, small rodents, moose etc. | Liver |
| Echinococcus multilocularis | IH: humans, cattle, swine, sheep, deer, horse, small rodents, moose etc. | Liver |
| Mesocestoides corti / M. lineatus | IH: dog, cat, other mammals, reptiles, rodents | Liver, peritoneal and pleural cavities, lungs, other organs |
| NEMATODES | ||
| Capillaria hepatica | DH: small rodents, rabbit, humans etc. | Liver |
| TREMATODES | ||
| Fasciola hepatica | DH: ruminants, dog, cat, horse | Bile ducts / Associated with Black disease and bacillary hemoglobinuria. |
| Fasciola gigantica | DH: cattle, sheep | Bile ducts |
| Fascioloides magna | DH: cattle, sheep, horse, pig | Liver |
| Dicrocoelid flukes | ||
| Eurytrema pancreaticum | DH: sheep, goat, cattle | Pancreatic and bile ducts and duodenum |
| Concinnum procyonis | DH: raccoon, fox, cat | Pancreatic and bile ducts |
| Dicrocoelium dendriticum | DH: sheep, goat, dog, pig, deer | Bile ducts / Associated with Black disease. |
| Dicrocoelium hospes | DH: cattle (Countries south to the Sahara) | Bile ducts |
| Platynosomum fastosum | DH: cat (North America and Amazonia) | Liver, bile ducts |
| Athesmia foxi | DH: New World monkeys | Bile ducts |
| Opisthorchid flukes | ||
| Metorchis albidus | DH: dog, cat, fox | Bile ducts |
| Metorchis conjunctus | DH: cat, dog, fox, mink (North America) | Bile ducts |
| Metorchis bilis | DH: red fox | Bile ducts |
| Opistorchis felineus | DH: cat, dog, fox (Europe, Russia) | Bile ducts |
| Opistorchis sinensis | DH: dog, cat, pig, humans, fox | Bile duct and duodenum. Associated with cholangiocarcinoma in humans. |
| Opistorchis tenuicollis | DH: dog, cat, fox, pig | Pancreatic and bile ducts |
| Pseudamphistomum truncatum | DH: dog, cat, fox | Bile ducts |
| Paramphistomatidae | ||
| Gigantocotyle explanatum | DH: cattle, buffalo | Bile ducts |
| Schistosomatidae | ||
| Schistosoma japonicum, S. mansoni, S. bovis, S. spindle etc. | DH: various species (humans, monkey, cat, dog, ruminants etc.) | Adults reside in veins (portal v., mesenteric v. etc.) |
JPC Diagnosis:
Conference Comment:
Aphasmid nematodes derive their name from the lack of a tiny set of sensory papillae (phasmids) on their caudal end, which are not identifiable histologically, but differentiate them from the phasmid nematodes (i.e. strongyles, rhabditoids, ascarids, oxyurids, rhabditoids and spirurids). Histologically visible characteristics that help distinguish them from the phasmids include absence of lateral cords, the presence of hypodermal/bacillary bands, and as mentioned above, the presence of a stichosome, which would only be seen in sections with esophagus. The eggs of some species are bipolar plugged / bioperculate as seen in this case, but may be embryonated or unembryonated depending on the specific parasite species.(4)
As mentioned above by the contributor, portal bridging fibrosis is a prominent feature of this entity. However, unlike many other types of portal fibrosis, hepatic stellate cells do not seem to participate in the pathogenesis of this lesion. Experimentally, septa are visible around the 23rd and 27th day after rat inoculation with embryonated eggs, and prominent angiogenesis precedes deposition of collagen, which is a key component in the development of hepatic fibrosis. The earliest changes are seen in portal areas, which may be located some distance away from the location of the actual nematodes,(7) and may emanate from multiple portal areas simultaneously.(3) In addition to angiogenesis, studies have found that proliferating cells responsible for the formation of prominent septa include fibroblast like cells(7) as well as pericytes and myofibroblasts.(3) Other cells, such as eosinophils, may also be present, but evidence for involvement of hepatic stellate cells is absent.(7) Interestingly, angiogenesis also seems to play a role in regression of fibrosis as it occurs in rats infected with C. hepatica.(3)
References:
1. Caswell JL: Tuberculosis. In: Maxie, MG ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Saunders Elsevier; 2007:606-610.
2. Ferreira LA, Andrade ZA. Capillaria hepatica: a cause of septal fibrosis of the liver. Mem Inst Oswaldo Cruz. 1993; 88: 441447.
3. Gaban L, Ramos CDL, Barbosa J+â°nior AA, Souza MM de, Andrade Z de A. Dynamics of Capillaria-hepatica-induced hepatic septal fibrosis in rats. Rev Soc Bras Med Trop. 2010; 43: 643646.
4. Gardiner CH, Poynton SL. An Atlas of Metazoan Parasites in Animal Tissues. Washington, DC: Armed Forces Institute of Pathology; 1999:40-43.
5. Gomes AT, Cunha LM, Bastos CG, Medrado BF, Assis BC, Andrade ZA. Capillaria hepatica in rats: focal parasitic hepatic lesions and septal fibrosis run independent courses. Mem Inst Oswaldo Cruz. 2006; 101: 895898.
6. Jones TC, Hunt RD, King NW. Diseases caused by parasitic helminths and arthropods. In: Veterinary Pathology. Wiley-Blackwell; 2007:601667.
7. Maria De Souza M, Tolentino M, Assis BCA, Cristina De Oliveira Gonzalez A, Maria Correia Silva T, Andrade ZA. Pathogenesis of septal fibrosis of the liver. (An experimental study with a new model). Pathol Res Pr. 2006; 202: 883889.
8. Mowat V, Turton J, Stewart J, Lui KC, Pilling AM. Histopathological features of Capillaria hepatica infection in laboratory rabbits. Toxicol Pathol. 2009 37: 661666.
9. Redrobe SP, Patterson-Kane JC. Calodium hepaticum (syn. Capillaria hepatica) in captive rodents in a zoological garden. J Comp Pathol. 2005; 133: 7376.