Signalment:
Gross Description:
Histopathologic Description:
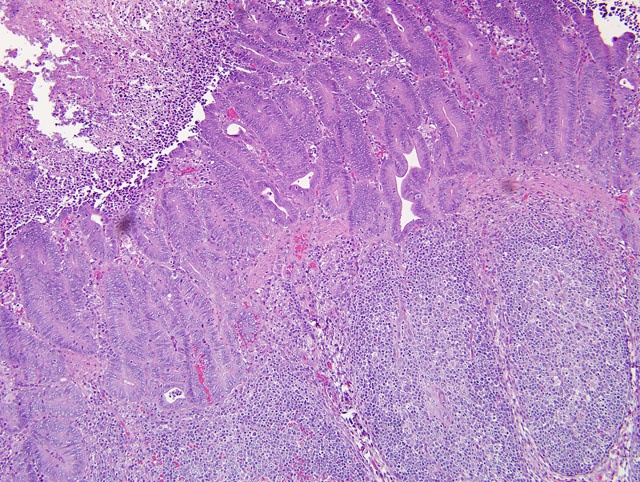
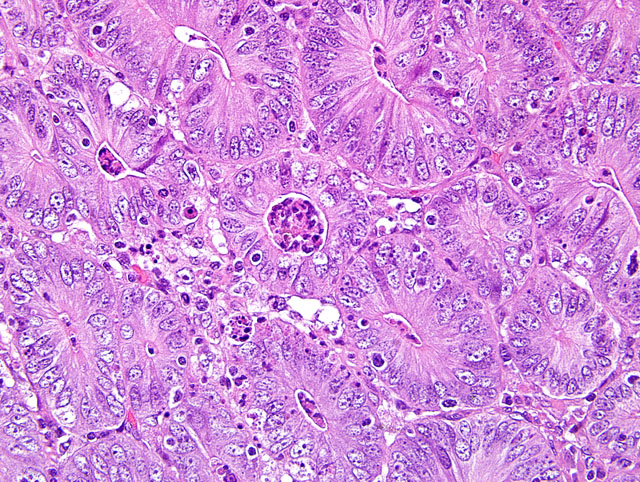
Diffusely, small intestinal villi are moderately to severely blunted and many are fused. Ileal segments have a thickened lamina epithelialis mucosae with long, branching crypts. The lamina propria mucosae has multifocal, mild, neutrophilic infiltrates. Peyers patches are moderately depleted and rarely have individual multinucleate giant cells. The colon has multifocal crypt necrosis and dilation and segmental ulceration with massive bacterial colonization. On Warthin-Starry silver impregnated re-cuts, large numbers of short, comma-shaped bacteria colonize the apical aspect of affected ileal enterocytes (Fig. 1 and 2).
Morphologic Diagnosis:
1. Ileum: Severe diffuse proliferative ileitis (with intraepithelial, commashaped bacteria as per special stain).
2. Small intestine: Moderate to severe diffuse villous atrophy.
3. Colon: Moderate multifocal, acute, fibrino-necrotizing colitis with intralesional colonies of mixed bacteria.
Lab Results:
Condition:
Contributor Comment:
1. The diffuse villus atrophy of the small intestine is characteristic of a viral infection. It is a bit surprising that a coronavirus was still detectable in this 10-week-old pig.
2. The proliferative ileitis and the morphology of the intraepithelial bacteria on silver impregnation are suggestive of an infection with either Lawsonia intracellularis or Campylobacter spp. The presence of L. intracellularis was confirmed by immunohistochemistry.
The nomenclature of this bacterial pathogen has been changed numerous times in the past decades, most recently from "ileal symbiont intracellularis to Lawsonia intracellularis.(9) Proliferative enteropathy (PE) with intraenterocytic L. intracellularis has been described in a wide range of hosts. The distribution of lesions varies with the host ranging from ileum (white tailed deer, horse, guinea pig, and rhesus macaques), to caudal ileum and colon (pig and hamster), cecum and colon (rabbit, blue fox, and ferret), to rectum (emu). Four forms of enteric lesions have been associated with L. intracellularis infection in pigs.(7) Weaners or young growing pigs are most commonly affected by a persistent uncomplicated proliferation sometimes described as porcine intestinal adenomatosis (PlA). The lower ileum and less commonly colon have small, raised, opaque islands to an irregular nodular or folded surface. Histologically, proliferating crowded immature enterocytes form branched and elongate crypts replacing the normal villous epithelium. Villus loss and the lack of a brush border on affected cells clearly interfere with intestinal physiology. Affected enterocytes are colonized by apical, comma-shaped bacteria that reside free in the cytoplasm. In mature animals (>4 months of age), infection results in proliferative hemorrhagic enteropathy (PHE), an acute clinical disease in which major intestinal hemorrhage arises from a proliferative ileal lesion. Proliferation may not be as severe as in PAI and bacteria may be seen also extracellular and in macrophages. Necrotic enteritis (NE) and regional ileitis (RI) represent a proliferative lesion that has been subject to further insult. Necrotic enteritis is an extensive coagulative necrosis of the epithelium that often results in rapid death. Regional ileitis is thought to be the outcome of NE with replacement of the damaged mucosa by granulation tissue associated with hypertrophy of the tunica muscularis ("hosepipe gut"). In a recent study on the infection dynamics of L. intracellularis under field conditions, shedding was detected by PCR in 75% of 100 pigs at 10-12 weeks of age (22-29 kg) and had ceased by 18 weeks of age. Seroconversion was detected after the expected lag time at 12-14 weeks of age and 92% of the pigs remained seropositive until slaughter.(11) A negative effect on growth rate was documented shortly before and during early infection followed by a compensatory impact. In another study, seroconversion and prevalence of intragastrically with L. intracellularis challenged piglets from L. intracellularis-seropositive and seronegative gilts were compared.(2) Piglets from seronegative gilts had highest seroprevalence at 5 weeks (84%) that declined gradually from week 11 to week 26 (10%). Offspring of seropositive gilts had only a seroprevalence of 23% at 5 weeks that decayed much faster to 0% at week 17. Cellular immune response to L. intracellularis infection has been documented in piglets challenged intragastrically with wild type and attenuated live vaccine by ELISPOT assay for IFN-gamma.(4)
Very little is known about the pathogenesis of L. intracellularis infection. This is largely due to the obligate intracellular nature of the pathogen, which does not allow application of standard molecular techniques.(8) An outer membrane protein LsaA (Lawsonia surface antigen) has recently been identified and partially characterized. It is a member of the TlyA family, which are proteins present in a wide range of bacteria that in a few cases cause hemolysis. LsaA does not mediate hemolysis, but is expressed during infection and monoclonal antibodies to LsaA significantly inhibit in vitro infectivity of L. intracellularis. This suggests a role of LsaA in attachment and/or entry of L. intracellularis. The site of attachment and entry in vivo are immature enterocytes at the base of crypts. How the proliferative character of the lesion comes about has yet to be determined. Some data suggests that bacterial replication and dissemination is tied to replication of the host cell. (data reviewed in7) L. intracellularis is difficult to culture (requires co-culture with mammalian cells). Histopathology in conjunction with demonstration of bacteria in tissues by silver impregnation can only suggest an infection with L. intracellularis as Campylobacter spp. share morphologic characteristics and can be encountered in enterocytes of proliferative enteritis cases in pigs. L. intracellularis can be identified by PCR on feces or diseased intestine; immunohistochemistry; or immunofluorescence on sections of affected intestine.(5,6)
3. The fibrino-necrotizing colitis is interpreted as a sequel of the proliferative enteropathy. The colonization with bacteria is thought to be a tertiary problem as a specific pathogen was not isolated. The differential diagnosis for fibrinonecrotic colitis should include salmonellosis and European [classical] and African swine fever. The significance of the detection of porcine circovirus type 2 (PCV) is uncertain, as it can be detected in clinically healthy pigs. The only lesion present that could be associated with a PCV2 infection in this piglet was the rare multinucleate giant cells in Peyers patches, yet they lacked cytoplasmic inclusion bodies.
JPC Diagnosis:
1. Ileum: Enteritis, proliferative, diffuse, moderate, with crypt necrosis and abscesses, and villar atrophy, blunting, and fusion.
2. Colon: Colitis, proliferative and necrotizing, diffuse, moderate, with crypt herniation.
3. Jejunum: Enteritis, lymphoplasmacytic, diffuse, moderate, with villar blunting and fusion.
Conference Comment:
Lawsonia intracellularis shares many features with other obligate intracellular pathogens in the orders Chlamydiales and Rickettsiales. Obligate intracellular bacterial pathogens often obtain a portion of their energy needs from the host cell; acquiring all of their metabolic demands from the host cell would be disadvantageous as this would reduce host cell proliferative potential. While both rickettsial and chlamyidal organisms are capable of generating their own energy, they also use the host cell as a supplementary source of energy, as well as a source of nucleotides. Based on genomic analysis, there is speculation that L. intracellularis can generate its own energy; however, the bacterium still requires supplementation from the host cell, and this is accomplished through the use of an ATP/ADP translocase whereby bacterial ADP is exchanged for host cell ATP. The ATP/ADP translocase enzyme belongs to a broad class of translocases termed nucleotide transport (NTT) proteins which import nucleotides or ATP from the host cell into the bacterium.(10)
In the past decade, L. intracellularis has been seen with increasing frequency in foals and weanlings. A retrospective study of infections in Kentucky between 2005 and 2007 found infection to be most common in two to eight-month-old thoroughbreds between the months of August and January. The most common clinical findings were ventral edema and hypoalbuminemia.(3)
References:
2. Barna P, Bilkei G. Effect of gilt seropositivity to Lawsonia intracellularis (LI) on their offsprings seropositivity to LI and on diarrhoea after a pure-culture challenge. Prev Vet Med. 2003;61:71-78.
3. Frazer ML. Lawsonia intracellularis infection in horses: 2005-2007. J Vet Intern Med. 2008;22:1243-1248.
4. Guedes RM, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135-145.
5. Guedes RM, Gebhart CJ, Winkelman NL, Mackie-Nuss RA, Marsteller TA, Deen J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can J Vet Res. 2002;66:99-107.
6. Huerta F, Arenas A, Carrasco L, et al. Comparison of diagnostic techniques for porcine proliferative enteropathy (Lawsonia intracellularis infection). J Comp Pathol. 2003;129:179-185.
7. Lawson GH, Gebhart CJ. Proliferative enteropathy. J Comp Pathol. 2000;122:77-100.
8. McCluskey J, Hannigan J, Harris JD, Wren B, Smith DG. LsaA, an antigen involved in cell attachment and invasion, is expressed by Lawsonia intracellularis during infection in vitro and in viva. Infect Immun. 2002;70:2899-2907.
9. McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820-825.
10.Schmitz-Esser S, Haferkamp I, Knab S, et al. Lawsonia intracellularis contains a gene encoding a functional rickettsia-like ATP/ADP translocase for host exploitation. J Bacteriol. 2008;190:5746-5752.
11.Stege H, Jensen TK, Moller K, Vestergaard K, Baekbo P, Jorsal SE. Infection dynamics of Lawsonia intracellularis in pig herds. Vet Microbiol 2004;104:197-206.
12.Turner JR. The gastrointestinal tract. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2009:801.