Signalment:
Unknown age and
gender, guinea pig, (
Cavia porcellus).The animal
developed a skin tumor of 1 cm in diameter at the right flank. The mass was
completely resected. Unfortunately, further clinical data were not available.
Gross Description:
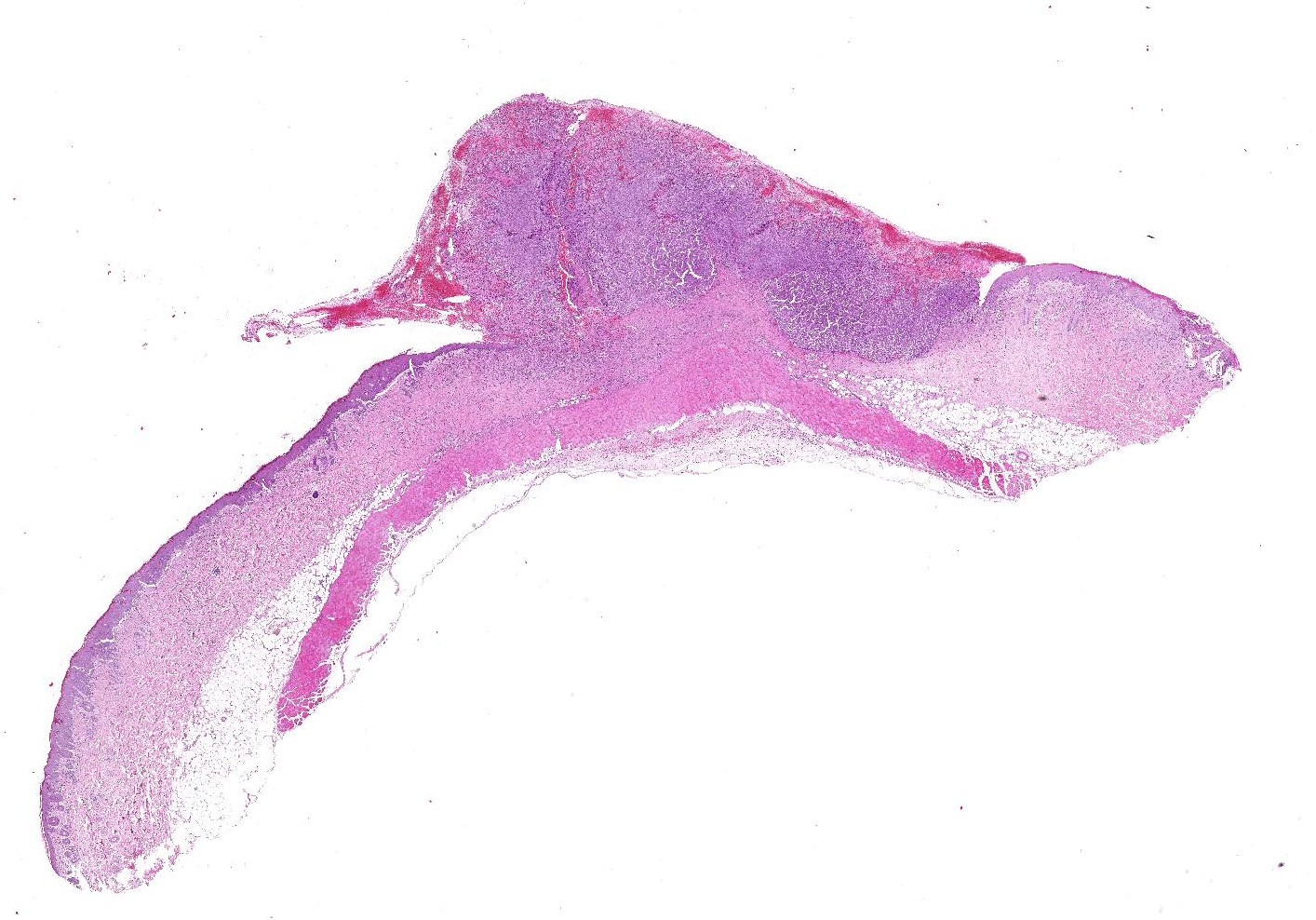
A 2 x 1
cm skin sample was submitted for histopathologic examination. Centrally there
was a well-demarcated, 1 x 1 cm partially exophytic firm nodule. The cut
surface was light brown.
Histopathologic Description:
Haired
skin: Elevating an ulcerated epidermis and infiltrating into the underlying
dermis (in some slides, tumor overlies an intact epidermis) is densely
cellular, well-demarcated, exophytic, partly infiltrative and ulcerated,
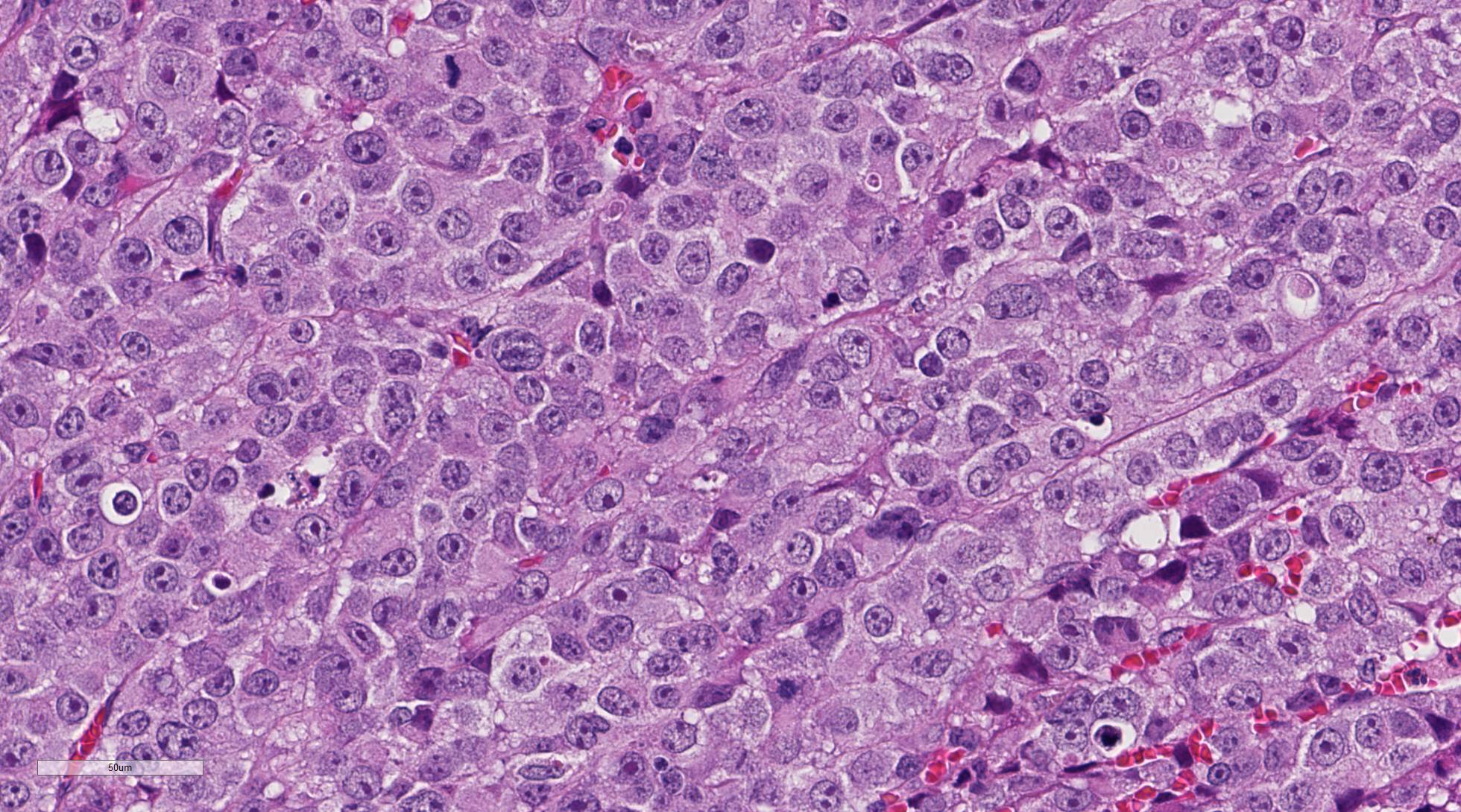
unencapsulated neoplasm composed of sheets of pleomorphic round cells within a
scant fibrovascular stroma. Neoplastic cells are round to polygonal, up to 40
um in diameter with variable distinct cell borders and moderate amounts of eosinophilic
cytoplasm. Nuclei are round to oval, centrally located with finely stippled
chromatin and up to four prominent magenta nucleoli. Mitoses average 2-3 per
high power field (some bizarre) and cells show moderate anisocytosis and
anisokaryosis. Occasionally, vascular invasion of tumor cells can be observed
(not in all slides). There are multifocal hemorrhages within and around the
tumor and hemorrhagic and serocellular crusts at the ulcerated surface. The
adjacent skin is hyperplastic with a moderate perivascular infiltration with
lymphocytes, plasma cells, and few heterophils.
Morphologic Diagnosis:
Haired skin: Amelanotic malignant melanoma, guinea
pig,
Cavia porcellus
Lab Results:
Immuno-histochemically
the neoplastic cells were positive for Melan-A and PNL2.
Condition:
Amelanotic melanoma
Contributor Comment:
Melanomas are
described in a variety of animal species including domestic animals and
wildlife species. However, they are most common in dogs, horses and some breed
of swine
10, only few reports of melanoma in birds, laboratory
animals and more recently in reptiles exist.
6,16 The histologic
diagnosis of melanoma is complicated due to variable degree of pigmentation and
the high variability of cell shapes. With the help of immunohistochemistry,
most amelanotic melanoma can be routinely diagnosed.
3,4,10
With our case,
we present a well-known neoplasm in an unusual species. Case reports of
spontaneous melanoma in the guinea pig are extremely rare, but the guinea pig
is a well-defined model of experimental melanoma using the potent carcinogen
7,12-dimethylbenz[
a]anthracene (DMBA), a polycyclic aromatic hydrocarbon.
7
This substance is proven to transform cells in different oncogenic pathways.
2
Mutations that
affect cell cycle control (p16/INK4a, CDK4), pro-growth pathways (growth factor
receptors, RAS, BRAF), and telomerase were identified in the patho-genesis of
malignant melanoma. Furthermore, melanomas can be inherited, and UV
light-induced DNA damage plays a role as do other factors.
8
The most common
naturally occurring skin tumors in guinea pigs are trichofolliculomas. They are
a subtype of trichoepithelioma and occur as expansible, often centrally cystic
neoplasia in the skin often of the lumbosacral region.
13,15,18 Other
tumors, like fibrosarcoma, lipoma, sebaceous gland adenoma, and hemangioma were
also reported in this species.
In this
melanoma, there is abundant vascularity. As observed in other species
5,12 the
amount of blood vessels may be a prognostic factor for this neoplasia in guinea
pigs, and particularly mast cells may play a significant role in angiogenesis
as the major source of VEGF.
1Unfortunately,
further information regarding the clinical course of the presented case was not
available.
JPC Diagnosis:
Haired skin:
Amelanotic melanoma, guinea pig,
Cavia porcellus.
Conference Comment:
Melanocytic neoplasms arise from melanocytes or melanoblasts which are derived
from the neural crest ectoderm. As mentioned by the contributor, melanomas have
been identified in most veterinary species and humans. The histologic diagnosis
of melanomas is sometimes complicated due to the variability of pigmentation
and arrangement of neoplastic cells into clear cells (balloon cell), spindle
cell, epithelioid cell, and signet ring cell histomorphology.
10
Additionally, there are often multiple different tumor cell morphologies within
a single neoplasm. For this reason, melanomas are sometimes referred to as one
of the great imitators due to their common embryologic connection with both
neural and epithelial origin.
10
This case nicely
demonstrates this point, due to variation in round to polygonal cellular appearance
across various regions of the neoplasm. Despite the rarity of melanocytic
neoplasms in guinea pigs and the lack of melanin granularity in this case, most
conference participants included amelanotic melanoma high within their
differential diagnosis. Prior to the conference, the Joint Pathology Center ran
the histochemical stain Fontana-Masson and immuno-histochemical stains melan-A
and S100. The Fontana-Masson stain highlighted multifocal positive argentaffin
granules and melanin within the cytoplasm of neoplastic cells. Additionally,
neoplastic cells are immunopositive for S100 and melan-A red, confirming the
diagnosis of an amelanotic melanoma, in this case.
As mentioned by the contributor, trichofolliculomas are the most common tumor
in the skin of guinea pigs; although, spontaneous neoplasms in this species are
rare in animals under three years old.
13 These benign dome-shaped
subcutaneous nodules, typically less than 2cm in diameter, most commonly occur
along the dorsal lumbar region and may represent a hamartomatous rather than a
neoplastic process.
10,13 Trichofolliculoma development is thought to
occur secondary to inhibition of bone morphogenic protein (BMP), an important
tumor suppressor gene.
13,14,18 Studies indicate that BMP plays a
critical role in maintaining homeostasis of hair follicles and regulation of
skin develop-ment.
14 In addition, BMP is an important growth factor
for a variety of tissues throughout the body and its con-centration is tightly
regulated in health. Interestingly, recent research in humans have shown that
absence of BMP signaling leads to the progression of colorectal carcinoma;
conversely, overexpression of BMP signaling induces epithelial-mesenchymal
transition, tumor invasion, and metastasis in a variety of malignant neoplasms.
9,11,14
References:
1. Ch'ng S, Wallis
RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies.
Mod
Pathol. 2006; 19(1):149-159.
2. Currier N,
Solomon SE, Demicco EG, Chang DL, Farago M, Ying H, Dominguez I, Sonenshein GE,
Cardiff RD, Xiao ZX, Sherr DH, Seldin DC. Oncogenic signaling pathways
activated in DMBA-induced mouse mammary tumors.
Toxicol Pathol.
2005;33(6):726-37.
3. Goldschmidt
MH, Dunstan RW, Stannard AA, Tscharner CV, et al. Histological
classification of epithelial and melanocytic tumors of the skin of domestic
animals.
Vol III. 2nd series. Washington D.C.: Armed Forces Institute of Pathology.
1998.
4. Goldschmidt MH,
Hendrick MJ. Tumors of the skin and soft tissue. In: Meuten DJ, ed.
Tumors
in Domestic Animals. 4th Ed. Ames, IA, USA: Blackwell Publishing;
2002:45-117.
5. Gregório H,
Raposo TP, Queiroga FL, Prada J, Pires I. Investigating associations of
cyclooxygenase-2 expression with angiogenesis, proliferation, macrophage and
T-lymphocyte infiltration in canine melanocytic tumours.
Melanoma Res.
2016; 26(4):338-347.
6. Heckers KO,
Schmidt V, Krastel D, Hildebrandt G, Kiefer I, Pees M. Malignant melanophoroma
in a Hermann's tortoise (
Testudo hermanni). A case report.
Tierarztl
Prax (K) 2011; 39:45-50.
7. Ingram AJ.
Review of chemical and UV light-induced melanomas in experimental animals in
relation to human melanoma incidence.
J Appl Toxicol. 1992;
12(1):39-43.
8. Lazar AJF,
Murphy GF. The skin. In: Kumar Abbas Aster Robbins and Cotran
. Pathologic
basis of disease. 9th Edition. Philadelphia, PA, Elsevier Saunders. 2015.
1147-1150.
9. Kan L, Liu Y, et
al. Inhibition of BMP signaling in P-cadherin positive hair progenitor cells
leads to tricho-folliculoma-like hair follicle neoplasms.
J Biomed Sci.
2011; 14:92.
10. Mauldin EA,
Peters-Kennedy J. Integumentary system. In: Maxie MG, ed.
Jubb, Kennedy and
Palmers Pathology of Domestic Animals. 6th ed. Vol 1. Elsevier, St. Louis,
Missouri; 2016:705,720-736.
11. Owens p, Pickup
MW, et al. Inhibition of BMP signaling suppresses metastasis in mammary cancer.
Oncogene. 2015; 34:2437-2449.
12. Pastushenko I,
Vermeulen PB, Carapeto FJ, Van den Eynden G, Rutten A, Ara M, Dirix LY, Van
Laere S. Blood microvessel density, lymphatic microvessel density and lymphatic
invasion in predicting melanoma metastases: systematic review and
meta-analysis.
Br J Dermatol. 2014; 170(1):66-77.
13. Percy DH,
Barthold SW.
Pathology of Laboratory Rodents and Rabbits, 4
th
ed. Ames, IA: Blackwell Publishing; 2016:251.
14. Sharov
AA, Mardaryev AN, Sharova TY, Grachtchouk M, Atoyan R, Byers HR, Seykora JT,
Overbeek
P, Dlugosz A, Botchkare VA. Bone
morphogenetic protein antagonist noggin promotes skin tumorigenesis via
stimulation of the Wnt and Shh signaling pathways.
Am J Path. 2009;
175(3):1303-1314.
15. Sommerey
CC, Köhler K, Reinacher M. Erkrankungen des Meerschweinchens aus Sicht der
Pathologie.
Tierarztl Prax (K) 2004; 32:377-383.
16. Thompson KA,
Campbell M, Levens G, Agnew D. Bilaterally symmetrical oral amelanotic melanoma
in a Boa constrictor (Boa constrictor constrictor).
J Zoo Wildl
Med.
2015; 46(3):629-32.
17. Voorneveld PW,
Kodach LL, et al. Loss of SMAD4 alters BMP signaling to promote colorectal
cancer cell metastasis via activation of Rho and ROCK.
Gastroenterology.
2014; 147:196-208.
18. Williams BH.
Non-infectious disease. III Guinea pigs. In: Suckow MA, Stevens KA, Wilson RP.
The
laboratory rabbit, guinea pig, hamster and other rodents. First Edition.
London, Waltham, MA, San Diego, CA. Elsevier, Academic press, 2012, 685-704.