CASE IV: WSC2017-18 Case 2 (JPC 4100655)
Signalment:
Adult female cynomolgus macaque (Macaca fascicularis).
History:
This animal was the positive control animal in an Ebola-Zaire vaccine study. All animals in the study were determined to be immunocompetent, confirmed by polyclonal lymphocyte activation in vitro, and seronegative for all retroviruses. The animal was inoculated intramuscularly with Ebola-Zaire and was euthanized six days later.
Research was performed under an Institutional Animal Care and Use Committee approved protocol in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in The Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where the research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Gross Pathology:
Upon gross examination, the following findings were recorded: mild reddening of the skin of the inner arms, face, and abdomen, diffusely tan and friable liver, mildly enlarged spleen, petechial hemorrhages of the urinary bladder mucosa, multifocal bilateral tan discoloration of kidneys, multiple reddened enlarged lymph nodes, and subcutaneous and intramuscular hemorrhage in the area of inoculation.
Laboratory Results:
None submitted.
Microscopic Description:
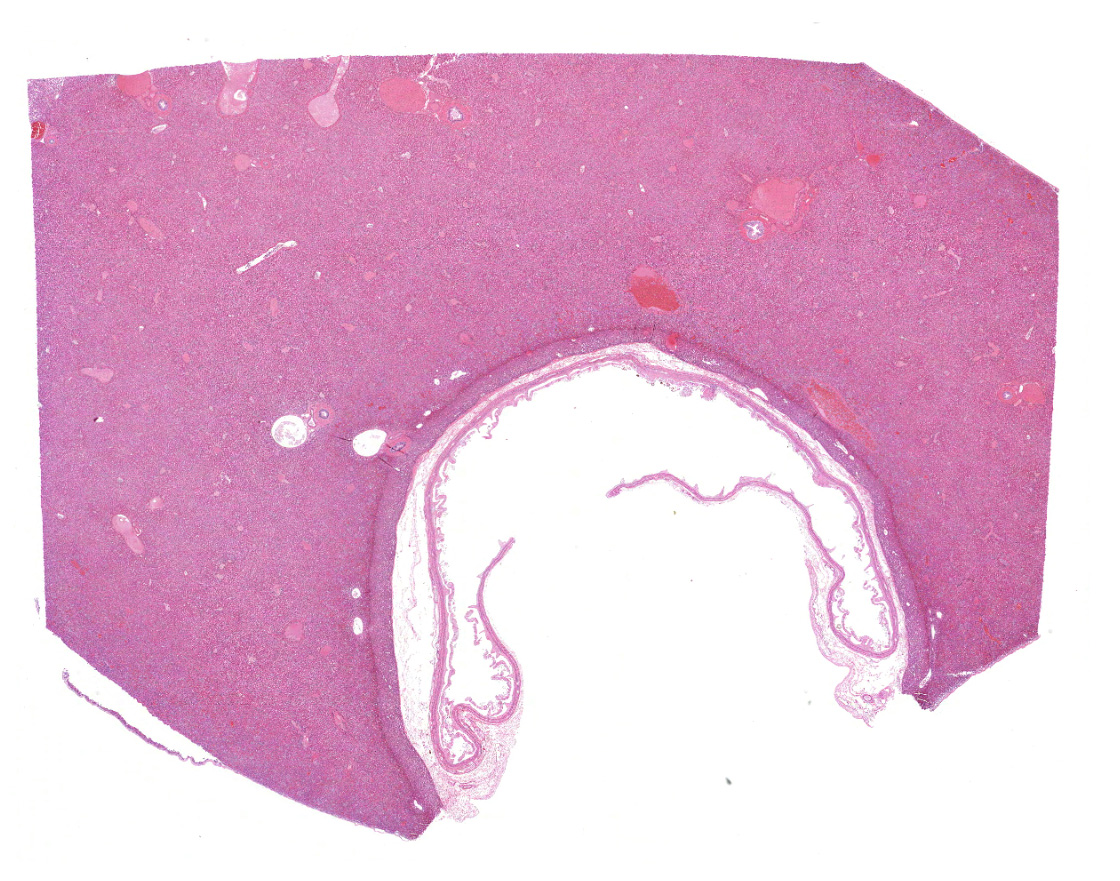
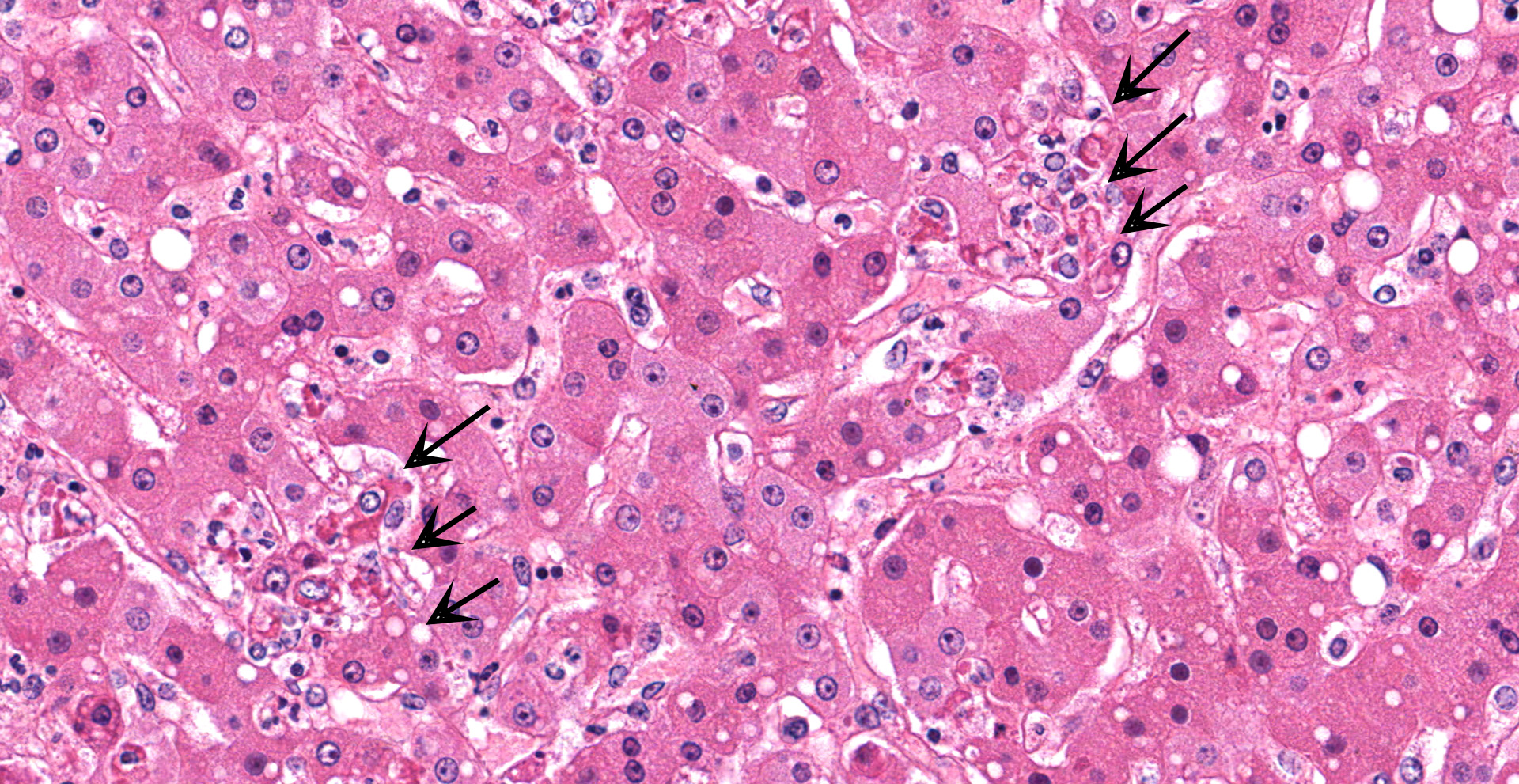
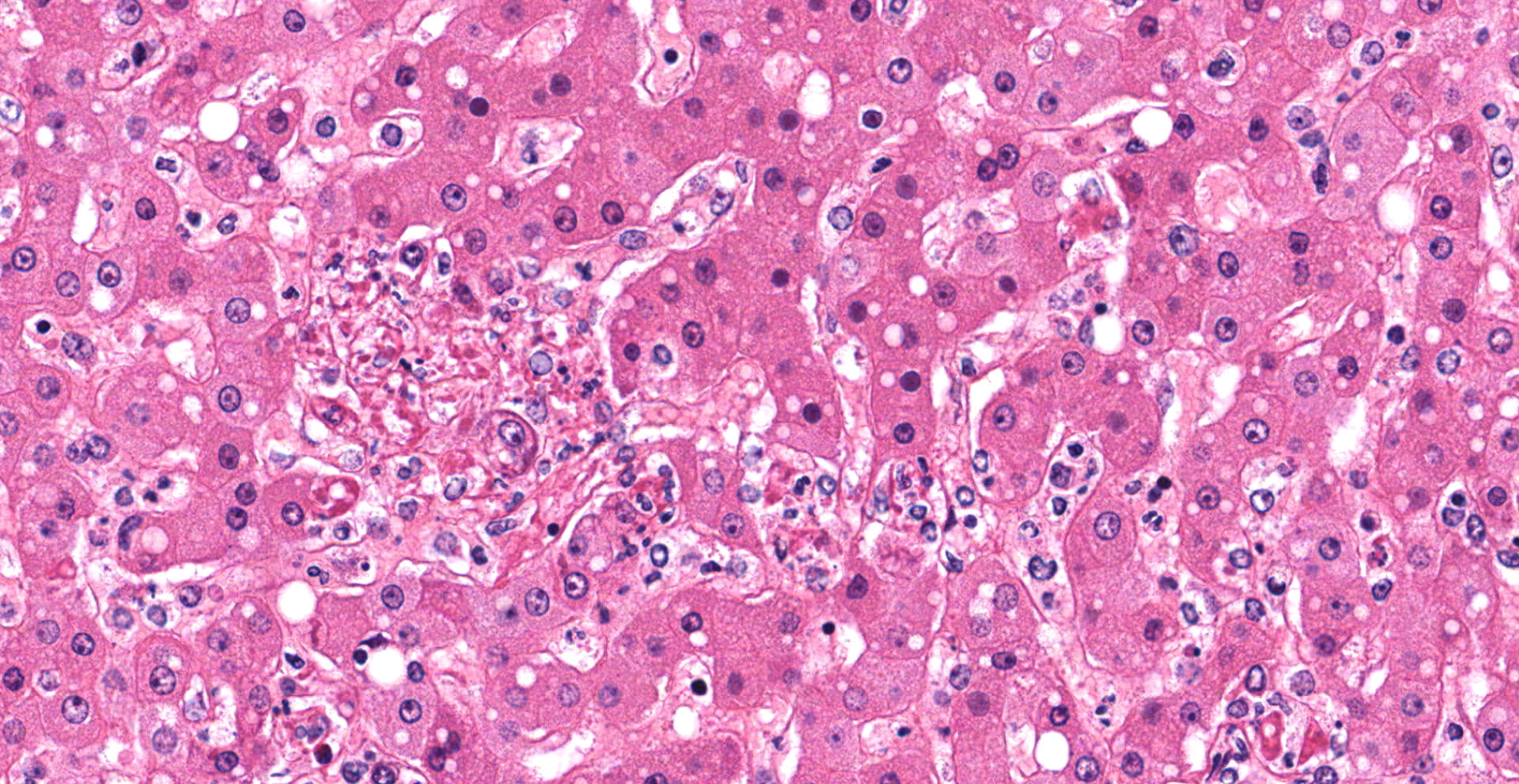
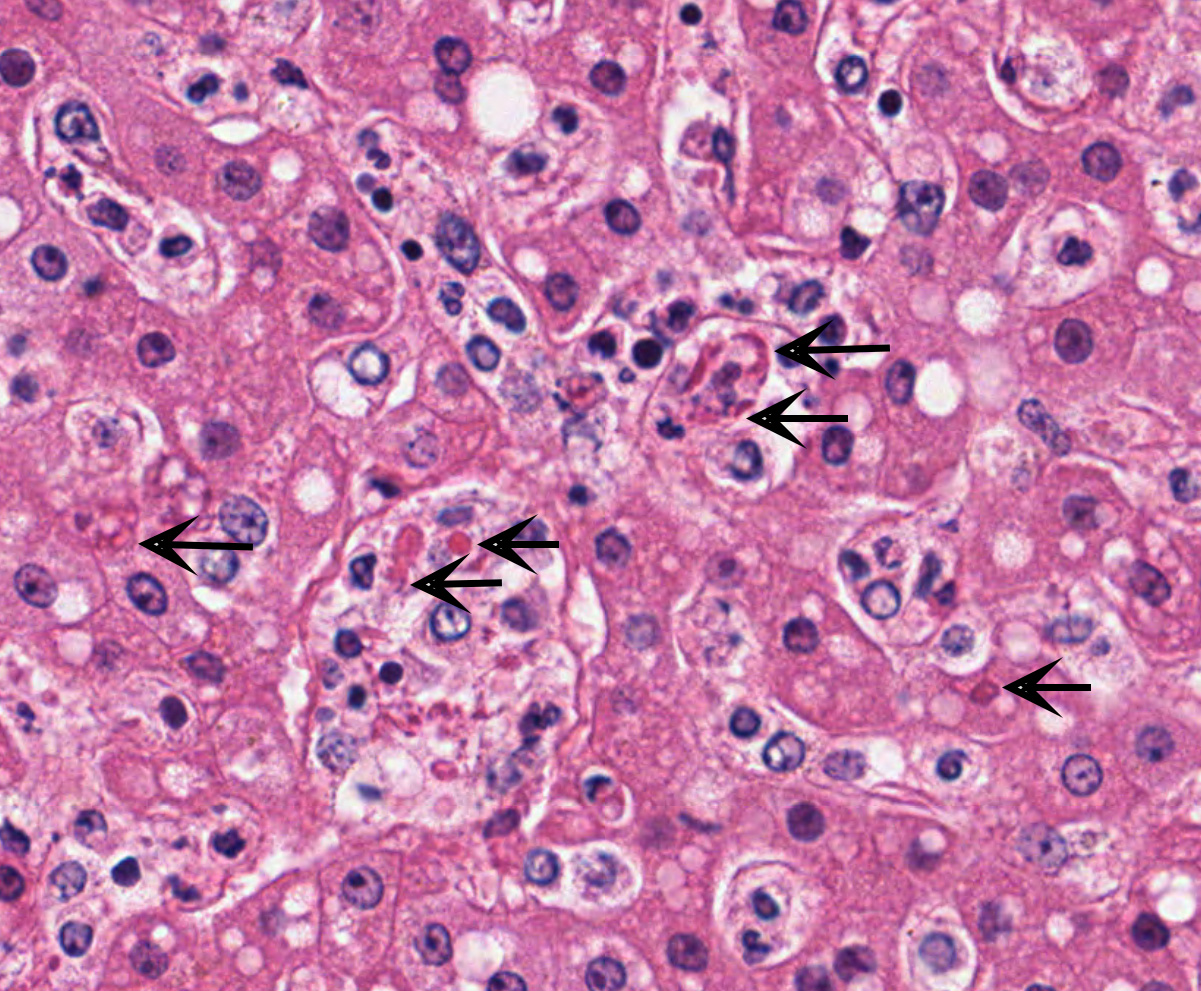
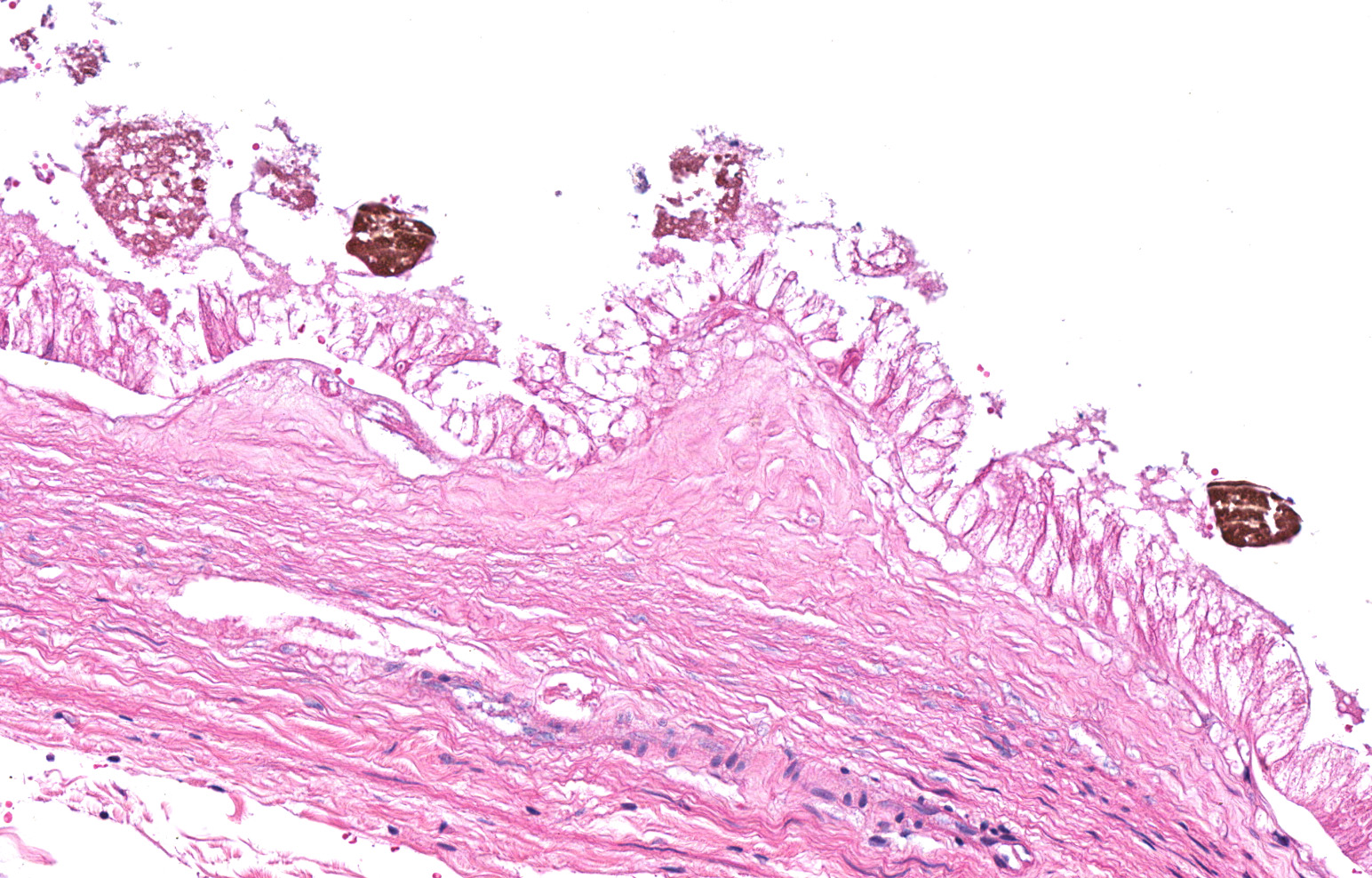
Liver with gallbladder: There are multifocal to coalescing, random areas of necrosis affecting approximately 25% of this section consisting of hepatocytes that are either individualized, shrunken, and hyper-eosinophilic (necrotic) or swollen with vacuolated cytoplasm (degenerate); and admixed with eosinophilic cellular and karyorrhectic debris, fibrin, and few neutrophils, lymphocytes and macrophages. Many hepatocytes, especially those within or adjacent to areas of necrosis, contain single or multiple variably sized, up to 7 um diameter, eosinophilic round to oval intracytoplasmic viral inclusion bodies. Diffusely, hepatic sinusoids are mildly expanded by fibrin and neutrophils. The majority of remaining hepatocytes contain a single or multiple discrete, clear lipid vacuoles which infrequently compress and peripheralize the nucleus. Multifocally, few arteries and veins contain fibrin, have a loss of integrity of the endothelial lining, and their
walls are transmurally infiltrated by neutrophils, lymphocytes, macrophages, necrotic cellular debris, fibrin, and hemorrhage (necrotizing vasculitis).
Contributor's Morphologic Diagnoses:
Liver: Hepatitis, necrotizing, multifocal to coalescing, moderate, with acute hepatitis, vasculitis, and hepatocyte intracytoplasmic viral inclusions.
Contributor's Comment:
The presented case demonstrates the typical hepatic necrotizing lesion of acute Ebolavirus (EBOV) infection in the Cynomolgus macaque. Inflammation is often absent to minimal. Cytoplasmic viral inclusions are most prominently identified within hepatocytes, although their presence in macrophages, to include within other infected organs, has been documented. Viral antigen is observed in many cell types in this case, to include hepatocytes, Kupfer cells, sinusoidal lining cells and endothelial cells. In comparison to the negative control for EBOLA antigen, there is strong positive immunoreactivity also within the serum diffusely within the sinusoids and blood vessels.
With regard to infectious diseases, few share the abilities of EBOV to cause global disruption, blanket international headlines, and strike fear into the world population as it does during epidemics. There have been 25 such epidemics since its discovery during simultaneous outbreaks in Zaire, Africa (now the Democratic Republic of the Congo) and Sudan in 1976.2 All have occurred along the equatorial belt of Africa, but the 2013 West Africa epidemic eclipsed them all as the most geographically extensive, fatal, and longest lasting in Ebola's history2; injecting a greater sense of urgency into the international community to identify therapeutics and vaccines.
EBOV is a Filovirus (single-stranded, negative-sense RNA virus), but is also a member of the viral group known to cause viral hemorrhagic fever.2,9 That group includes Dengue, Lassa, and Yellow Fever among others; and while all emerge from different reservoirs with variable pathogenesis, they all feature severe systemic viral infection associated with hemorrhagic phenomena such as petechiae, ecchymoses and frank bleeding.2 There are 5 species of EBOV, Bundibugyo, Reston, Sudan, Tai Forest and Zaire.9 All but Reston are pathogenic to humans.1 Zaire EBOV is the most common cause of epidemics and also the culprit of the most recent one in West Africa.2
Both Rhesus and Cynomolgus macaques are considered the gold standard model of EBOV disease for their consistent similarity to human disease.2 Nonhuman primates (NHP) may play a role in the natural history of EBOV, but no mammalian reservoir has yet been identified and most recent studies implicate fruit bats.6,7 Following an unknown route of exposure from a reservoir species to a human or NHP, viral transmission between these species occurs via inoculation into the bloodstream or exposure to mucus membranes or nonintact skin.2 Aerosol transmission has been described experimentally but never recorded in humans and only described in one study in NHP's.9 NHP's develop fever, diarrhea and macular rash along with leukocytosis, lymphopenia, thrombocytopenia and elevated D-Dimers typically within 6 days post-inoculation.3 Fatality rates are greater than 90% in Macaca spp., and death invariably occurs between 7-10 days post infection.3
The hallmark lesions of EBOV in NHP's include petechial and/or ecchymotic hemorrhages of the mucus membranes, internal organs, and skin; widespread lymphocytolysis in the lymph nodes and spleen; splenic fibrin deposition of the red pulp; hepatic necrosis; hepatocytic viral inclusions; and hemorrhage and congestion of the submucosa throughout the gastrointestinal tract, most prominently in the duodenum.3 Ocular lesions of necrotizing scleritis and conjunctivitis have been described in recovering Rhesus Macaques which parallels similar late onset lesions in humans.1,2
EBOV initially targets dendritic cells and macrophages which then disseminate to lymphoid tissues where viral replication occurs. The virus quickly becomes widely disseminated in the bloodstream; and multiple organs, especially the liver and spleen, are targeted as multiple cell types within these organs to include endothelial cells become infected. Lymphocytes are the rare cell type that avoids viral infection.2
The major mediators of EBOV tissue damage and disease manifestation include proinflammatory cytokines (TNF-α, IL-1, IL-6 & MCP/MIP), nitric oxide, tissue factor, increased TRAIL or Fas-FasL expression, and the EBOV glycoprotein.2 Tissue damage is further enhanced by ischemia; a culminating effort from tissue factor activation, fibrin thrombus formation, hepatic necrosis, and consumption of platelets and clotting factors. Late-appearing hemorrhages are thought to represent disseminated intravascular coagulation.2
EBOV induces a robust immune response capable of clearing infection in all but immunologically privileged organs (eye, testis, etc.). When the infection is identified early and supportive medical care is available, survival can be expected for many if not most patients. The case-fatality ratio among those 27 patients treated in Europe or the United States during the last epidemic was just 18.5%, stark contrast to the 40% range among those patients remaining in West Africa or the more historical 88% fatality rate for the earliest outbreaks.2 Targeted therapies may have contributed to this success; replication inhibitors, plasma infusion, and a recent cocktail of three monoclonal antibodies known as ZMapp have all been shown experimentally to reduce mortality in mice and/or NHP's.2 Documented success attributed to these has been less fruitful among humans thus far, however.
While no vaccine has yet been licensed, there are currently 11 different EBOV vaccines undergoing clinical study. Four are in phase III testing, and one focusing on the EBOV glycoprotein has gained widespread recognition due to documentation of 100% efficacy in a ring vaccination trial during the recent West Africa epidemic.4 Much of the natural history and pathogenesis of EBOV remains an enigma, however. Even if an effective vaccine becomes commercially available, the economic and logistical challenges of the endemic regions will likely continue to necessitate their rapid deployment following an epidemic emergence until more is understood regarding the underlying causes which may contribute to their prevention altogether.
Contributing Institution:
US Army Medical Research Institute of Infectious Diseases (USAMRIID)
Fort Detrick, MD
https://riid-vision.detrick.army.mil/pathology
JPC Diagnosis:
Liver: Hepatitis, necrotizing, random, multifocal, severe, with vasculitis and numerous intracytoplasmic viral inclusions.
JPC Comment:
The contributor provides a thorough and outstanding review of Ebolavirus (EBOV), the second filovirus reviewed by the Wednesday Slide Conference in three weeks (see 21-22 WSC Conf. 17-1 Marburgvirus).
Since the submission of this case in 2017, two vaccines for Zaire EBOV have been licensed: Ervebo and the two dose vaccine regimen of Zabdeno and Mvabea.10
Ervebo is a single dose vaccine that utilizes an attenuated-live recombinant stomatitis virus-based vector that expresses the envelope glycoprotein (GP) gene of Zaire EBOV.7 Consequentially, Ervebo has not been demonstrated to provide protection against disease caused by filoviruses other than Zaire EBOV.9 Regarding the 2-dose vaccine regimen, Zabdeno is monovalent replication incompetent adenoviral vector vaccine that encodes the full length GP of the Mayinga variant of Zaire EBOV. The second dose, Mvabea, is a multivalent Modified Vaccinia Ankara virus vaccine that similarly encodes for the GP of the Mayinga variant of Zaire EBOV, in addition to the Gulu GP of Sudan EBOV, Musoke GP of Marburgvirus, and the nucleoprotein of Tai Forest EBOV.8
Ervebo was first utilized under a "compassionate use" protocol for 16,000 people in Guinea during a 2015 EBOV outbreak, with similar utilization during the 2018-2020 outbreak in the Democratic Republic of Congo with the immunization of 345,000 individuals. Ervebo was licensed in November 2019 by the European Medicines Agency, followed shortly thereafter by the United States Food and Drug Administration, as well as in multiple West African nations. Due to limited quantities of available vaccine, Ervebo is not utilized in a traditional manner with mass vaccination campaigns. Instead, the vaccine is maintained as part of a strategic stockpile reserved for outbreak response focused on establishing a "ring vaccination" strategy, similar to the approach used to eradicate smallpox. Notably, "ring vaccination" does not imply vaccination of individuals within a specific geographic area following identification of a positive case. Instead, extensive contact tracing is used to identify individuals with a high risk of exposure, such as those in close contact with an infected person's body, body fluids, linen, or clothes over a 21 day period. Historically, each positive case is typically associated with approximately 150 individuals deemed to be at high risk of exposure.10
In May 2020, the European Medicines Agency recommended market authorization for the two dose vaccine regimen of Zabdeno and Mvabea for persons >1 year of age. The first vaccine in the protocol, Zabdeno, is followed approximately 8 weeks later by the second vaccine, Mvabea. Given the prolonged amount of time between the initial vaccination and immunity, the Zabdeno Mvabea 2-dose vaccine regimen is less suitable for outbreak responses than Ervebo. In addition, a booster vaccination of Zabdeno is recommended for individuals at imminent risk of exposure to EBOV (e.g. healthcare workers and those living or visiting areas with an ongoing outbreak) if more than four months have passed since administration of the second dose.10
Although vaccines are powerful tools for curbing future EBOV outbreaks, they are only one of many components of an effective control strategy. Additional components include early detection of new EBOV infections, functional laboratories to confirm infections, isolation and provision of supportive care for patients, and safely (albeit respectfully) burying the dead in a timely manner to reduce further spread through contact with deceased patients. 9
As discussed in WSC#17, EBOV and Marburgvirus are both reported to persist in immune-privileged tissues of survivors, including the aqueous humor, central nervous system, and testicles. Sexually transmission of both viruses has been reported. In one case, Ebola virus RNA was detected by reverse transcription PCR in semen 531 days following onset of symptoms.3 The moderator used this fairly recent discovery as an example to underscore the importance of collecting samples from as many tissues as possible when operating in a research setting, even those deemed inconsequential at the time of collection. If subsequent discoveries warrant further investigation, the availability of these tissues for retrospective analysis may not only provide more timely information, but also facilitate subsequent study design and/or reduce the need for additional resources, such as animal models. This practice is of particular importance in studies utilizing NHPs given their limited availability.
References:
1. Alves DA, Honko AN, Kortepeter MG, et al. Necrotizing scleritis, conjunctivitis, and other pathologic findings in Ebola virus-infected rhesus macaque (Macaca mulatta) with apparent recovery and a delayed time of death. J Infect Dis. 2016;213(1):57-60.
2. Baseler L, Chertow DS, Johnson KM, Feldmann H, and Morens D. The pathogenesis of ebola virus disease. Annu Rev Pathol Mech. 2017;12:387-418.
3. Den Boon S, Marston BJ, Nyenswah TG, Jambai A, Barry M, Keita S, et al. Ebola Virus Infection Associated with Transmission from Survivors. Emerg Infect Dis. 2019 Feb;25(2):249-255
4. Geisbert TW, Hensley LE, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques. Am J Pathol. 2003;163(6):2347-2370.
5. Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV- vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857-866.
6. Leendertz SJ, Gogarten JF, Dux A, Calvignac-Spencer S, Leendertz FH. Assessing the evidence supporting fruit bats as the primary reservoirs for Ebola viruses. Ecohealth. 2015;13:18-25.
7. Olival KJ, Hayman DT. Filoviruses in bats: current knowledge and future directions. Viruses. 2014;6:1759-1788.
8. Tomori O, Kolawole MO. Ebola virus disease: current vaccine solutions. Curr Opin Immunol. 2021;71:27-33.
9. Twenhafel NA, Mattix ME, Johnson JC, et al. Pathology of experimental aerosol Zaire Ebolavirus infection in rhesus macaques. Vet Pathol. 2012;50(3):514-529.
10. World Health Organization. Ebola virus disease: Vaccines. World Health Organization. Retrieved February 14, 2022, from https://www.who.int/news-room/questions-and-answers/item/ebola-vaccines.