Signalment:
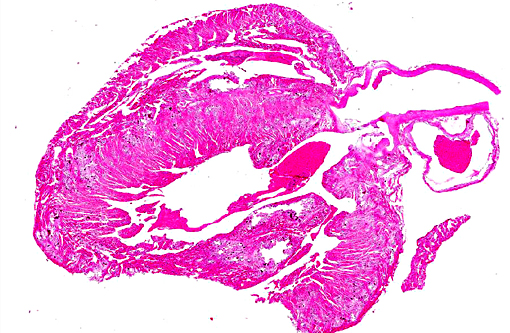
Gross Description:
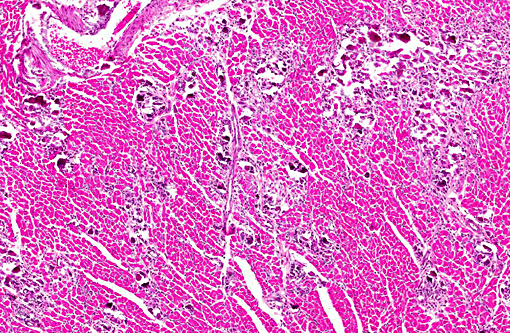
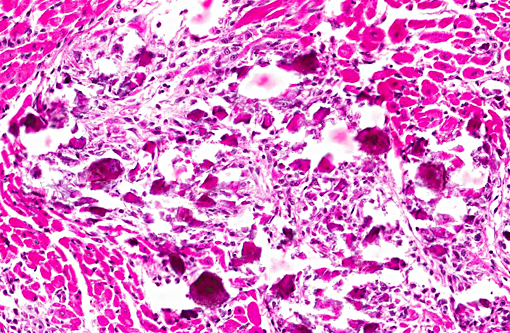
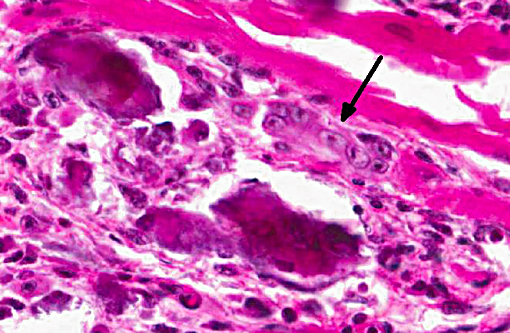
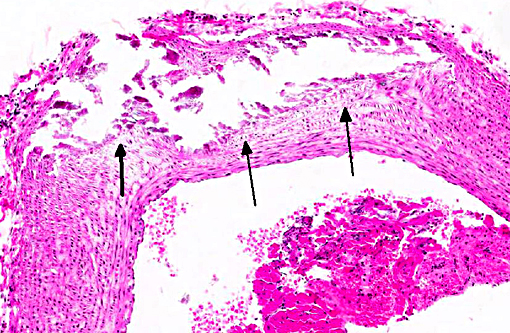
Histopathologic Description:
Mineralization of hepatocytes and renal tubular epithelium was also observed, and rarely the lung and spleen were mineralized (not submitted). In some animals there was centrilobular atrophy of the liver and hemosiderin laden alveolar macrophages (heart failure cells) in the lungs.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The major action of vitamin D (1,25(OH2)D3) is to increase calcium and phosphorus absorption from the intestine via increased synthesis of calbindin by enterocytes.(1) Vitamin D also acts on the bone, stimulating osteoclastic resorption and making osteocytes responsive to parathyroid hormone. Vitamin D can stimulate calcium resorption in the kidney as well.
The major differential etiology for these lesions is metastatic mineralization, a poorly understood and likely multifactorial disease predominantly affecting male guinea pigs >1 year of age.(3,7) Diet, particularly when high in phosphate and low in magnesium, is reported to be a significant factor. Only 4% of 140 animals examined in the Sparschu and Christie study had demonstrable cardiac mineralization, but lesion appearance and ventricular localization was similar (but less severe) than that reported herein. It is interesting to note that the guinea pigs in that study were given kale, a vitamin D rich plant.
The epizootic nature of the cases here combined with the young age (14 weeks) of one of the guinea pigs suggested the probability of vitamin D toxicosis. Plants high in vitamin D glycosides include day blooming jessamine (Cestrum diurnal), Trisetum flavescens, and members of the Solanum (nightshade) family including S. malacox-ylon.(1) The latter agent produces the disease enteque seco or espichamento in Argentina and Brazil. In addition to diet, accidental or deliberate rodenticide poisoning should be ruled out.
JPC Diagnosis:
1. Heart, myocardium: Myocyte, degeneration, necrosis, atrophy and loss, multifocal to coalescing, severe with marked fibrosis, granulomatous myocarditis and mineralization.
2. Aorta: Mineralization, intimal and mural, multifocal, moderate.
Conference Comment:
Gross lesions described by Holcombe et al. in a colony of guinea pigs similarly affected included white discoloration of multiple tissues including the gastrointestinal tract, skeletal muscle, and kidneys in addition to the cardiac muscle. Histologic lesions included varying degrees of mineralization in the tissues described grossly, as well as the lungs. Fibrosis and granulomatous inflammation were associated with mineralization, as seen in this case. Bone lesions were also demonstrated in affected guinea pigs, including osteosclerosis (most prominently in the tibia), and changes in the articular cartilage. Additional lesions described in those cases included fatty change in the liver and chronic interstitial nephritis. Clinicopathologic changes included hyperphosphatemia and elevated serum alkaline phosphatase. Serum calcium levels (ionized and total) were not significantly elevated in affected guinea pigs, and are not a reliable indicator of vitamin D status in the guinea pig. However, the values of calcium multiplied by that of phosphorus was greater than 70 for the majority of affected animals in that study.(4)
Conference participants described degeneration, necrosis and loss of cardiac myocytes secondary to mineralization. Participants discussed the differential diagnoses for metastatic calcification including renal disease, neoplasia such as lymphoma, multiple myeloma and tumors affecting the parathyroid gland resulting in primary hyperparathyroidism, as well as vitamin D toxicosis. Diagnostic techniques for investigating an outbreak of nutritional or toxic disease case involving multiple animals were also a point of discussion.
References:
1. Capen CC. Endocrine glands. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Vol 2. 5th ed. Edinburgh: Elsevier Saunders; 2007:325-428.
2. Craig LE, Dittmer LE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 1. 6th ed. St. Louis, MO: Elsevier; 2016:89.
3. Galloway JH, Glover D, Fox WC. Relationship of diet and age to metastatic calcification in guinea pigs. Lab Anim Care. 1964; 14: 6-12.
4. Holcombe H, Parry NM, Rick M, Brown DE, et al. Hypervitaminosis D and metastatic calcification in a colony of inbred strain 3 Guinea pigs (Cavia porcellus). Vet Pathol. 2015; 52(4): 741-751.
5. Jensen JA, Brice AK, Bagel JH, Mexas AM, Yoon SY, Wolfe JH. Hypervitaminosis D in guinea pigs with alpha-mannosidosis. Comp Med. 2013; 63:156-162.
6. Joseph DR. The Ratio between the heart-weight and body-weight in various animals. J Exp Med. 1908; 10: 521-528.
7. Sparschu GL, Christie RJ. Metastatic calcification in a guinea pig colony: a pathological survey. Lab Anim Care. 1968; 18: 520-526.