WSC 22-23
Conference 9
Case I
Signalment:
11½ -year-old, spayed female, American Bulldog, canine (Canis lupus familiaris)
History:
Two years ago, this canine presented for routine examination and recent increased urination. Ultrasound confirmed mass-like lesions at the trigone and the neck of the urinary bladder. The attending veterinarian suspected transitional cell carcinoma at the time. The owners elected for conservative treatment, so the canine was started on nonsteroidal anti-inflammatory drugs (NSAIDs), famotidine, omeprazole, and gabapentin.
Four months ago, the canine started coughing intermittently. Radiographs revealed multiple chest masses with metastatic pattern and osteophytes of the left distal humerus, suspicious of an early metastatic lesion.
A month later, the canine presented with lameness on the right front paw. Radiographs revealed periosteal reaction and sclerosis in the 2nd metacarpal bone. Not long after, euthanasia was elected due to pain on the right front limb and poor quality of life.
Gross Pathology:
This is the carcass of a 31 kg spayed over-conditioned female canine with no autolysis.
The lungs have a dozen variable-sized firm masses, ranging from 1 cm scattered throughout to a large 12x10 cm obliterating 50% of the caudal left lobe. The masses are bulging, well demarcated and off-white pale to reddish in color. They are distending and replacing the normal pulmonary parenchyma. The mediastinal lymph nodes are enlarged (1 cm x 5 cm) and flat in shape. On cut section, there are small white nodules (0.3 cm).
The kidneys are within normal limits. The urinary bladder mucosa at the level of the trigone has a soft, 1 cm thick, off-white, fuzzy irregular layer that covers 5 cm around the trigone extending into the urethra. The fundus of the bladder has three masses up to 1.5 cm in diameter that are firm, nodular and protruding.
Other lymph nodes, right axillary, mesenteric and retroperitoneal, are firm and slightly enlarged.
The liver has a diffuse, mild reticular pattern with occasional white pale well-demarcated foci (up to 0.5 cm).
The free edges of the left atrioventricular valve have multiple variable-sized nodules (1 mm) with a smooth surface.
The second right metacarpal bone has a 3 cm in diameter hard mass that extends into the proximal phalanx and the distal carpal bone. Upon opening the bone, the mass is necrotic and extends and effaces the carpal bone.
Laboratory Results:
No findings reported.
Microscopic Description:
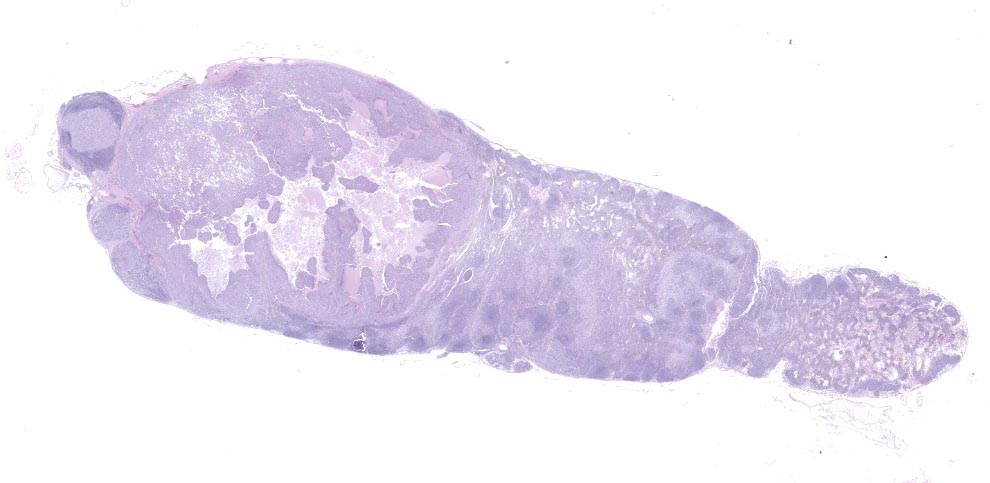
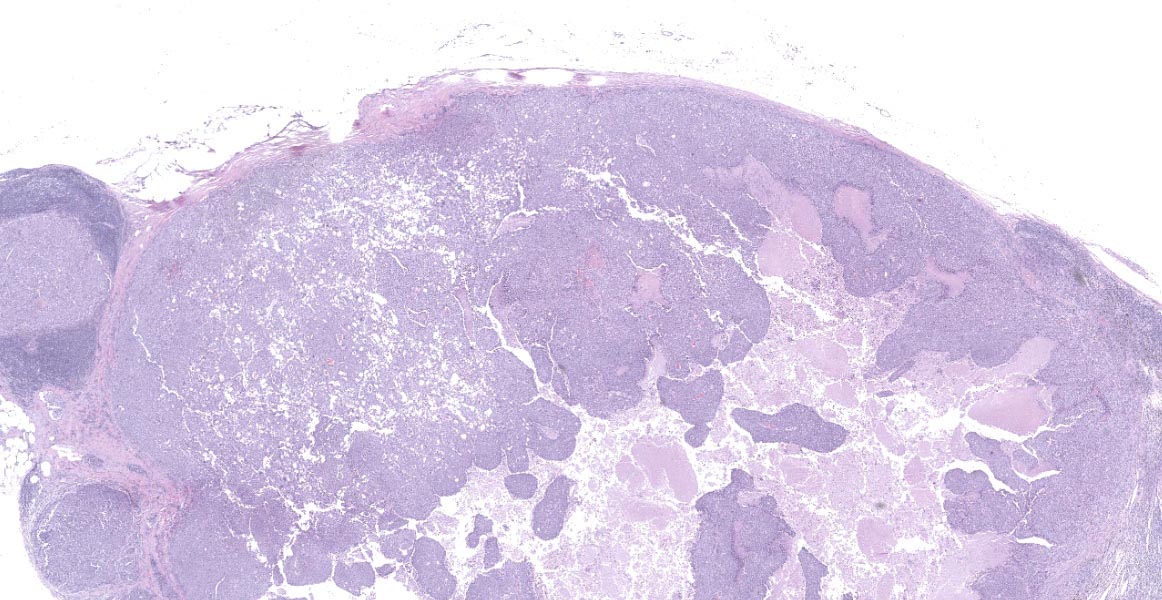
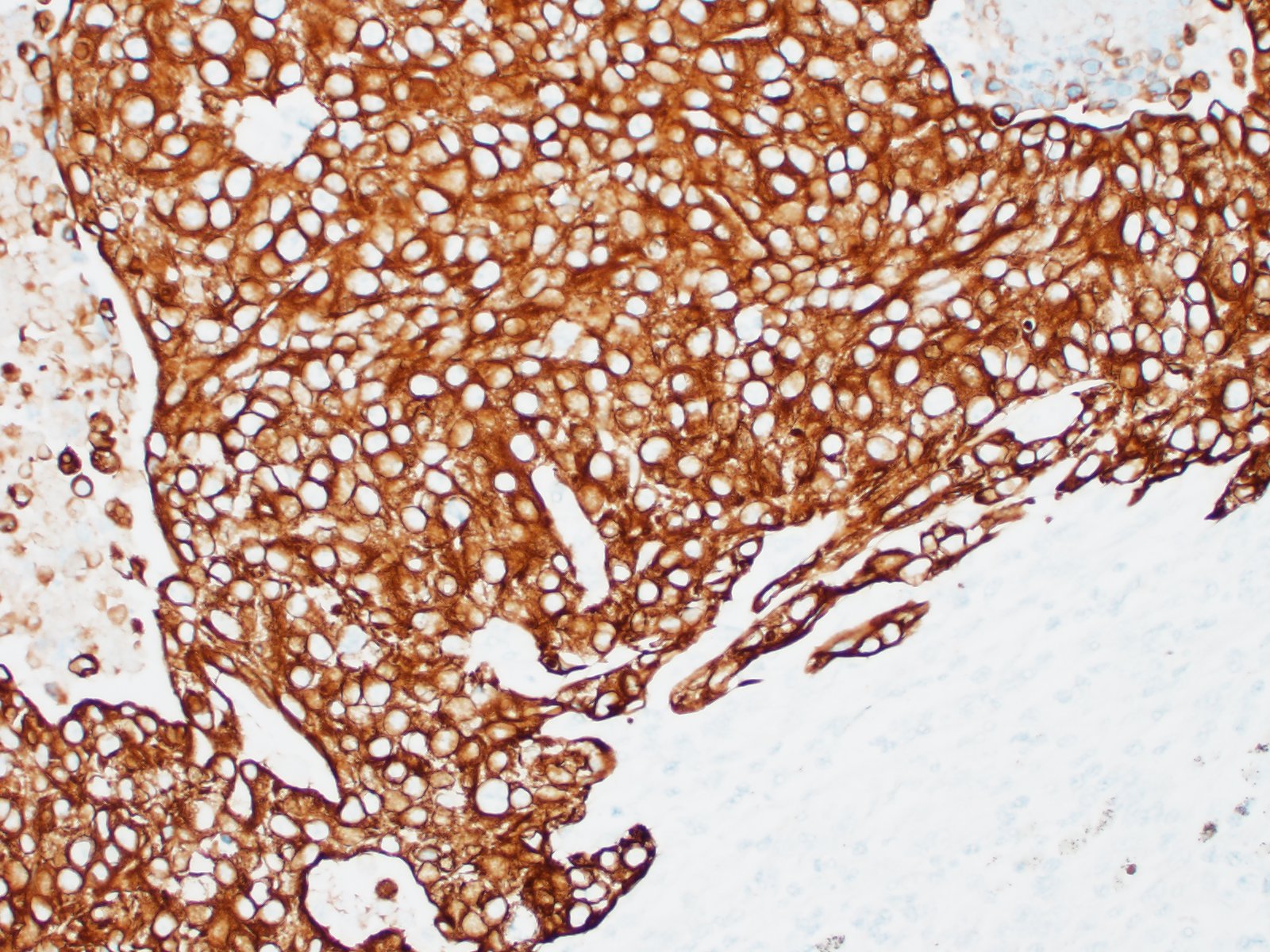
Lymph node- mediastinal: there is a well-demarcated not encapsulated expansile nodule that effaced and replaced the normal follicular lymphoid architecture. The nodule is composed of fronds or thick undulating ribbons of tightly packed neoplastic cells supported by a fine fibrovascular stroma.
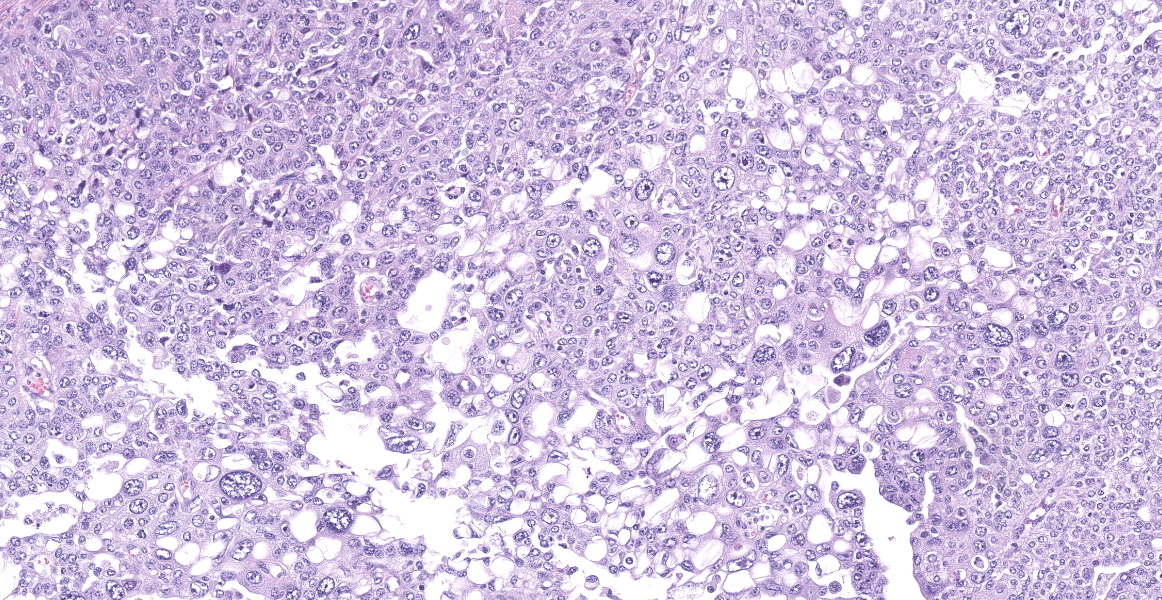
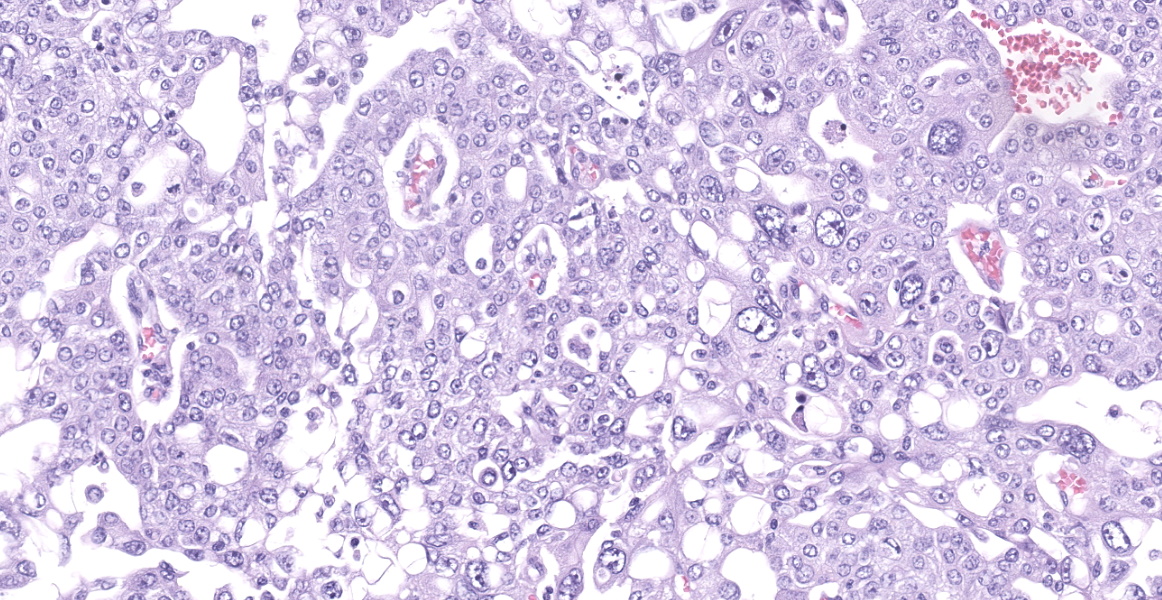
Neoplastic epithelial cuboidal to polyhedral cells with abundant pale eosinophilic cytoplasm. The cells show moderate anisokaryosis and anisocytosis with occasional megalokaryosis. There are areas where the cells are less compacted and the cytoplasm has variable-sized empty vacuoles. Occasionally, the large vacuoles displace the nucleus to the periphery. Some of the vacuoles occasionally contain a homogenous granular material. The core of the neoplastic nodule has a large necrotic area.
There are 20 mitotic figures in 10HPF (40x), with a moderate number of aberrant mitotic figures.
There are small nodules of neoplastic cells in the subcapsular sinuses. These nodules are present only in some of the sections.
Contributor’s Morphologic Diagnoses:
Lymph node: metastatic transitional cell carcinoma/urothelial carcinoma
Contributor’s Comment:
Transitional cell carcinoma (TCC), or urothelial carcinoma (UC), is a malignant tumor that originates in the transitional epithelium of the urinary tract. Squamous and glandular carcinomas can also occur. The urinary bladder is the most common site of the lesion, but it can occur anywhere form the renal pelvis to the distal urethra. Within the urinary bladder, TCC is most commonly diagnosed in the trigone area. TCC mostly occurs in cats and dogs. Bladder neoplasms are rare in horses, sheep, goats, and pigs.
Carcinomas in the urinary bladder can be classified in broad groups: Urothelial carcinoma, squamous cell carcinoma, adenocarcinoma, or undifferentiated carcinoma. This is based on the predominant cell type or the organization of the lesion. Urothelial carcinomas (UC) can be further classified based on
their patterns of growth as papillary (project into the lumen), flat, degree of anaplasia (graded on a scale of 1-4), and degree of infiltration (infiltrating or noninfiltrating). Most TCCs are intermediate to high-grade papillary infiltrative tumors.
TCC/UC tend to occur in older dogs (average 9-11 years). There is an approximate 2:1 ratio of female: male for bladder neoplasm but this is not always a consistent finding. However, neutered dogs seem to be predisposed to bladder neoplasms. Some breeds, like Scottish terriers. Airedales, Shetland sheepdogs, West Highland white terriers, fox terriers or beagles, have a high risk for TCC/UC than other dogs.
Of all the canine cases of TCC/UC, 90% demonstrate clinical signs. These signs include hematuria, pollakiuria, cystitis or dysuria. These urinary system clinical signs are not unique to neoplasms. Other clinical signs that are not related to the urinary system are mostly due to metastasis. These can include lameness due to bone metastasis or hypertrophic osteopathy, or dyspnea due to pulmonary metastasis.
Most of the literature suggests that tumors of the urinary bladder are less common in cats than in dogs. Cats with TCC/UC are usually older (6-18 years). Most of the clinical signs relate to the lower urinary tract: hematuria, stranguria, dysuria, and pollakiuria. Current urinary tract infection is present in over 70% of the cats with TCC.
If TCC/UC is suspected, the diagnostic work-up should include complete blood count, serum biochemistry profile, urinalysis, urine culture, radiographs (thorax and abdomen), and bladder imaging. Urine should be collected only by free catch or bladder catheterization. Ultrasound is useful to assess the bladder wall thickness but also for viewing regional lymph nodes and other abdominal organs for metastases. A diagnosis of TCC/UC requires histopathologic confirmation.
It can be difficult to differentiate tumors in the prostatic region of male dogs as urothelial origin or prostatic origin. About 30% of TCC/UCs are in the prostate and in neutered dogs it is the most common prostatic tumor. This should be carefully considered when determining the origin of a neoplasia in the prostatic region. Factors that favor TCC/UC Melamed-Wolinska bodies, UPIII immunoreactivity, CK7 immunoreactivity, desmoplasia, and wide-spread metastases.
High grade, invasive TCC/UCs are among the most malignant neoplasms and cause death via metastasis and cachexia. Upon diagnosis in canine patients, about 20% have detectable pulmonary metastases, 15% have lymph node metastases, and 6% have lumbar or pelvic bone metastases. In addition, squamous or glandular metaplasia may be important in the prediction of metastases.
Prognosis of dogs with TCC/UC is grave. Less than 20% of treated dogs live more than 1 year.
In humans, most of the cancer associated death, approximately 90%, are caused by metastatic disease rather than primary tumor. The effort to further characterize the neoplastic dissemination has expanded the research in carcinogenesis. First, to understand the metastatic dissemination behind this process, a sequence of a multi-step process of invasion is proposed: the metastatic cascade: 1) Epithelial primary neoplastic cell invasion into the surrounding stroma tissue and extra cellular matrix; 2) Epithelial cell intravascular invasion; 3) Neoplastic epithelial cells need to survive during circulatory transit. 4) Neoplastic cell arrest and extravasation through vascular walls and into the parenchyma or distant tissue; 5) Formation of microneoplastic colonies. Second, in addition to the metastatic cascade, the neoplastic cells need to be transformed and the concept of epithelial mesenchymal transition (EMT) has been expanded to further characterize carcinogenesis. EMT is a complex biological process where the epithelial cells acquire new properties to successfully invade tissue. These properties include 1) increased motility invasion and 2) ability to degrade extracellular matrix. These EMT properties show at different levels depending on the tissue site and the degree of malignancy. EMT is orchestrated by different tissue transcription factors starting with EMT-inducing transcription factors (EMT-TFs) such as SNAIL, SLUG, or ZEB1. There is still controversial evidence about how much these biological process are involved in metastasis. The other current metastatic process is the dormant niche, where dormant disseminated tumor cells will reside within the site of stem cells and somehow will be protected.
Primary and metastatic tumors of the bladder occur in dogs and rarely in cats; several tumor types have been reported. Hematuria is a common sign. Metastatic bladder neoplasia is rare but some of the signs listed below are due to metastases. It has been associated with hypertrophic osteoarthropathy. Systemic signs of urinary obstruction are seen if urine flow at the trigone is blocked. Tumor cells might be present in the urine. Benign polyps of the urinary bladder induce clinical, laboratory and radiographic characteristics similar to neoplasia of the bladder.
Transitional cell carcinoma (TCC) is the most common cancer of the bladder in dogs. Tumors most commonly originate in the trigone, but they can also occur or extend through the urethra. The most common metastatic sites are the iliac and other abdominal lymph nodes, liver and lung. Ultrasonography of the abdomen is done to measure the size of the tumor and look for metastases within the abdomen. When the tumor is accessible, surgery is the best option to prolong survival for dogs with TCC when followed by chemotherapy. Regardless of whether surgery is possible or not, chemotherapy has been shown to alleviate symptoms and prolong survival for many dogs with TCC.
Contributing Institution:
https://www.westernu.edu/veterinary/
JPC Diagnosis:
Lymph node: Urothelial carcinoma, metastatic.
JPC Comment:
Recently, a urine assay measuring the BRAFV595E mutation was developed that provides clinicians with a non-invasive means of differentiating cystitis from neoplasia.3,8 The BRAF assay has a high sensitivity and sensitivity for the mutation, which affects approximately 70% of dogs with urothelial carcinoma.3,8 The BRAFV595E mutation in dogs is analogous to the BRAFV600E mutation in many human cancers. The mutation causes constituent activation of the MAPK pathway, preventing apoptosis of neoplastic cells and increasing invasiveness and metastasis.8 A few recent studies found no significant difference in survival times in UCs with and without the BRAFV595E mutation; the mutation does, however, make the neoplasm more amenable to treatment. Dogs with BRAFV595E mutation treated with chemotherapy or both chemotherapy and surgery had double or triple the survival times, respectively, compared to dogs treated with NSAID therapy alone.5
In dogs, the BRAF mutation has also been associated with increased CCL17 expression, which is significantly higher than levels in healthy dogs or dogs with non-neoplastic urinary disease.8 This is due to overexpression of COX-2 by neoplastic cells, which stimulates production of PGE-2 and upregulation of CCL17. 8 The end effect of CCL17 elevation is infiltration of Foxp3+ regulatory T cells (T-reg) within and surrounding the neoplasm.8 In many cancers, T-regs are associated with a poor prognosis, possibly due to inhibition of antitumor immune activity.8
The overproduction of COX-2 by neoplastic cells has made NSAIDs, such as peroxicam, one of the main therapeutic agents used to prolong UC survival times.4,8 A recent study found LOX-5 was similarly overproduced by comparing immunohistochemistry scores of UC, cystitis, and normal bladder mucosa.4 These results suggest that NSAIDs with anti-LOX-5 activity may provide another target for prolonging survival times and should be investigated further.4
References:
- Anesi S, Parry AT, Monti P, Elliott J. Radiographic appearance of an osseous metastasis to the distal radius from a transitional cell carcinoma of the urinary bladder. Veterinary Record Case Reports. 2017; 5(4): p.e000474.
- Epstein JI, Lotan TL. The Lower Urinary Tract and Male Genital System. In: Kumar V, Abbas AK, Aster JC, eds: Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier Saunders. 2015: 976-981.
- Ettinger S. Urine luck: A new test for canine bladder and prostate cancer. July 29, 2019. Accessed October 16, 2020. https://www.dvm360.com/view/urine-luck-new-test-canine-bladder-and-prostate-cancer
- Finotello R, Schiavo L, Ressel L, Frohmader A, Sivlestrini P, Verin R. Lipoxygenase-5 Expression in Canine Urinary Bladder: Normal Urothelium, Cystitis and Transitional Cell Carcinoma. J Comp Path. 2019; 170:1-9.
- Gedon J, Kehl A, Aupperle-Lellbach H, von Bomhard W, Schmidt JM. BRAF mutation status and its prognostic significance in 79 canine urothelial carcinomas: A retrospective study (2006-2019). Vet Comp Oncol. 2022; 20(2):449-457.
- Knapp DW, McMillan SK. Tumors of the Urinary System. In: Withrow SK, Vail DM, Page RL, eds: Withrow & MacEwen’s Small Animal Clinical Oncology. St. Louis, MO: Elsevier Saunders. 2013; 572-582.
- Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017; 168(4): 670-691.
- Maeda S, Yoshitake R, Chambers JK. BRAFV595E Mutation Associates CCL17 Expression and Regulatory T Cell Recruitment in Urothelial Carcinoma of Dogs. Vet Pathol. 2021; 58(5): 971-980.
- Marvel SJ, Séguin B, Dailey DD, Thamm DH. Clinical outcome of partial cystectomy for transitional cell carcinoma of the canine bladder. Veterinary and Comparative Oncology. 2017; 15(4): 1417-1427.
- Meuten DJ, Meuten TLK. Tumors of the Urinary System. In: Meuten DJ, ed: Tumors in Domestic Animals. 5th Ames, IO: John Wiley & Sons, Inc. 2017; 666-675
- Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annual Review of Pathology: Mechanisms of Disease. 2018; 13: 395-412.
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011; 147(2): 275-292.