Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 25
6 May, 2020
CASE II: MU11478 (JPC 4115990). Tissue from a horse (Equus caballus).
Signalment: A 20 day-old female brown and white goat (Capra aegagrus hircus).
History: This goat had a weeklong history of lameness in the left front leg. It improved slightly after a dose of banamine and florphenicol and then lameness progressed. At the time of euthanasia, there was swelling and clinical lameness in both carpal joints. The goat was nursing poorly, and the owner requested euthanasia on humane grounds.
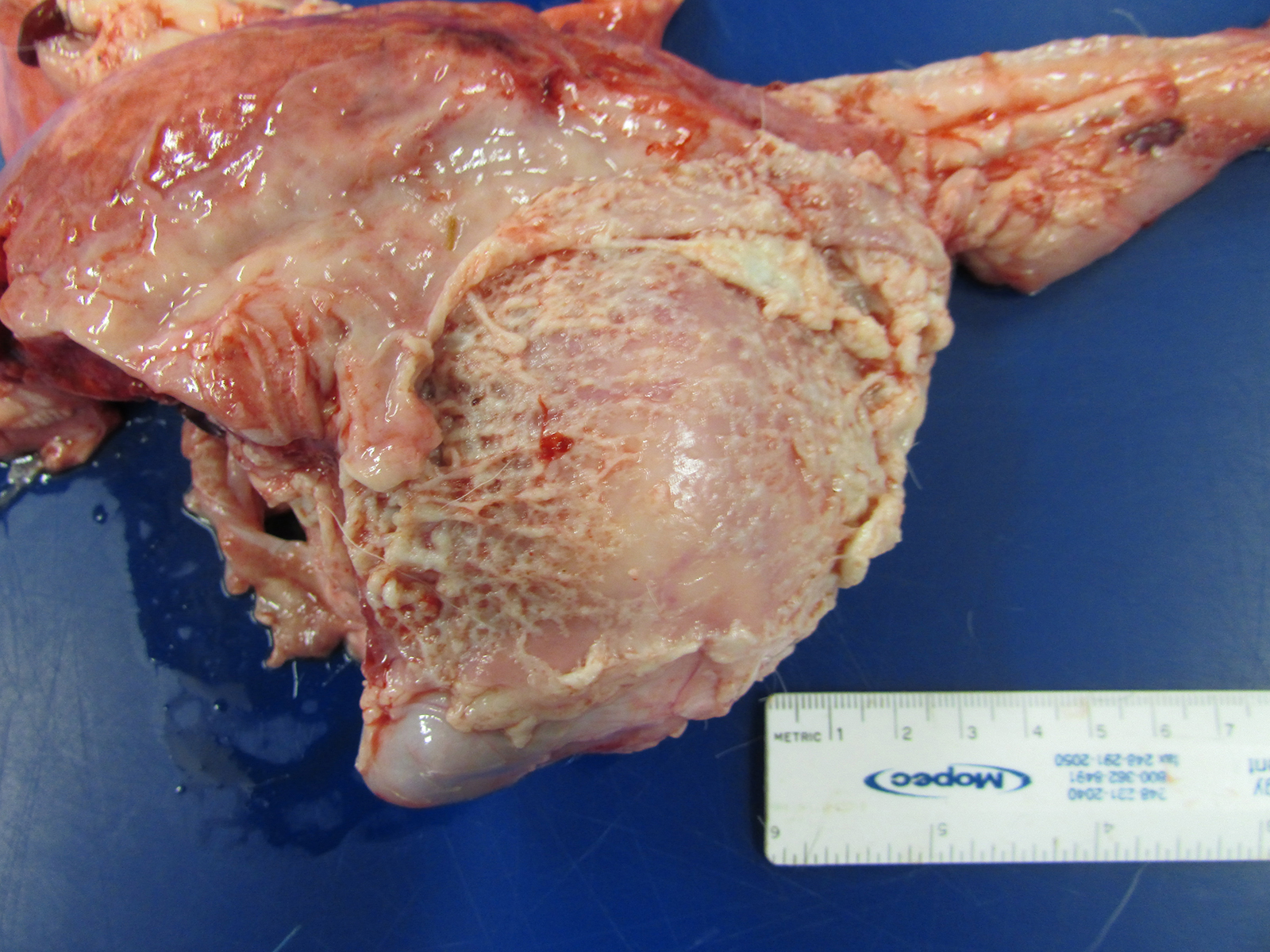
Gross Pathology: The goat kid weighed 5.7 Kg at necropsy. Lesions were limited to the joints. In addition, there was marked, tan to white, caseous to fibrinous exudate, with increased synovial fluid volume, expanding multiple joints and multifocally infiltrating into the surrounding extracapsular tissue. The left and right shoulders, carpi, coxofemoral, right stifle, and right tarsal joints were affected.
The thoracic cavity contained marked, multifocal, yellow-tan fibrinous exudate that was multifocally adhered to the pleura, body wall, and pericardium, with numerous easily broken adhesions. The lungs are multifocally tan and red, mildly firm, and float in formalin.
Laboratory results: Small colonies of bacteria were isolated on sheep blood agar from swabs of the right carpus, right shoulder and left fetlock. Polymerase chain reaction identified them as Myocoplasma mycoides subsp. capri.
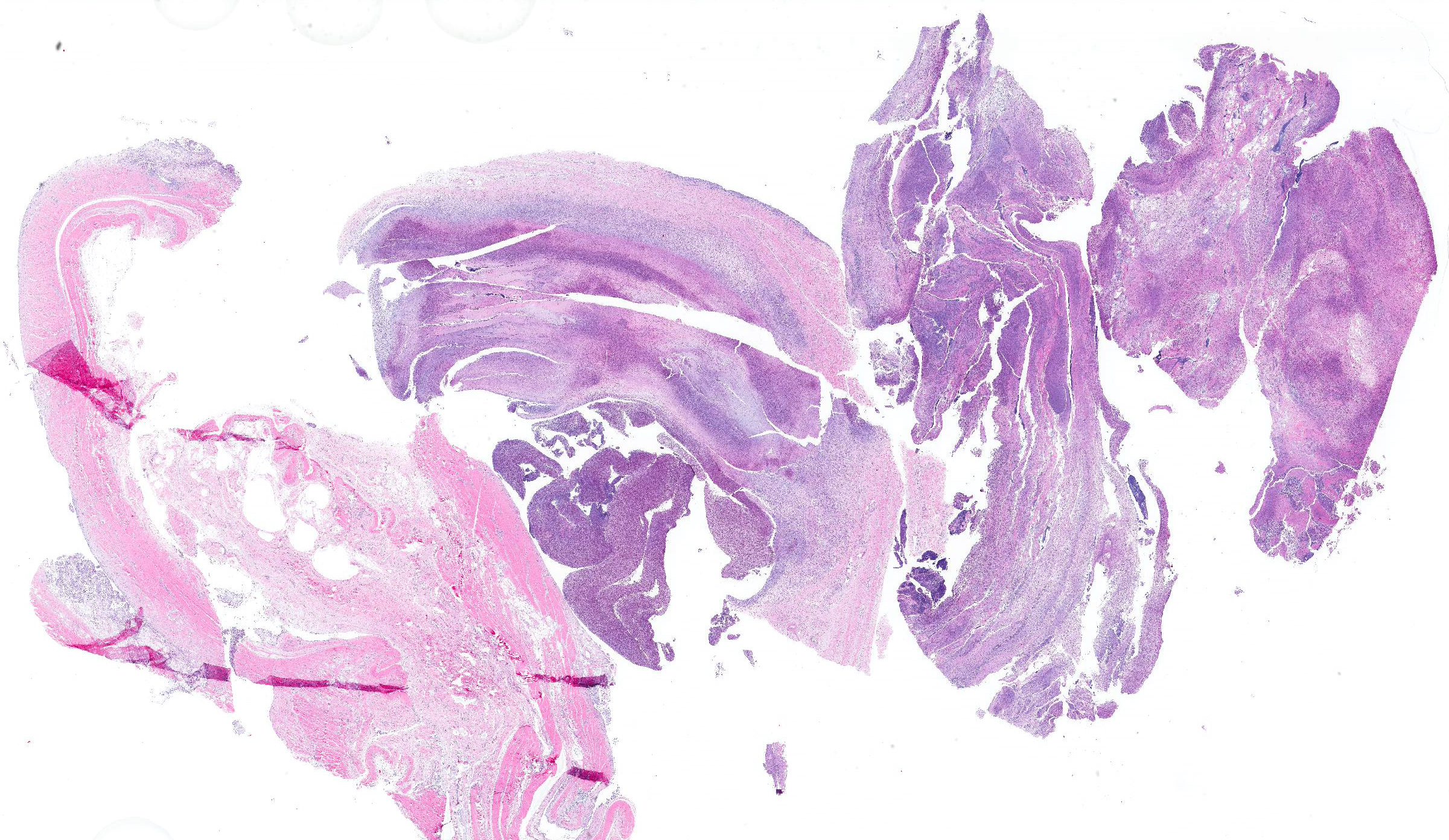
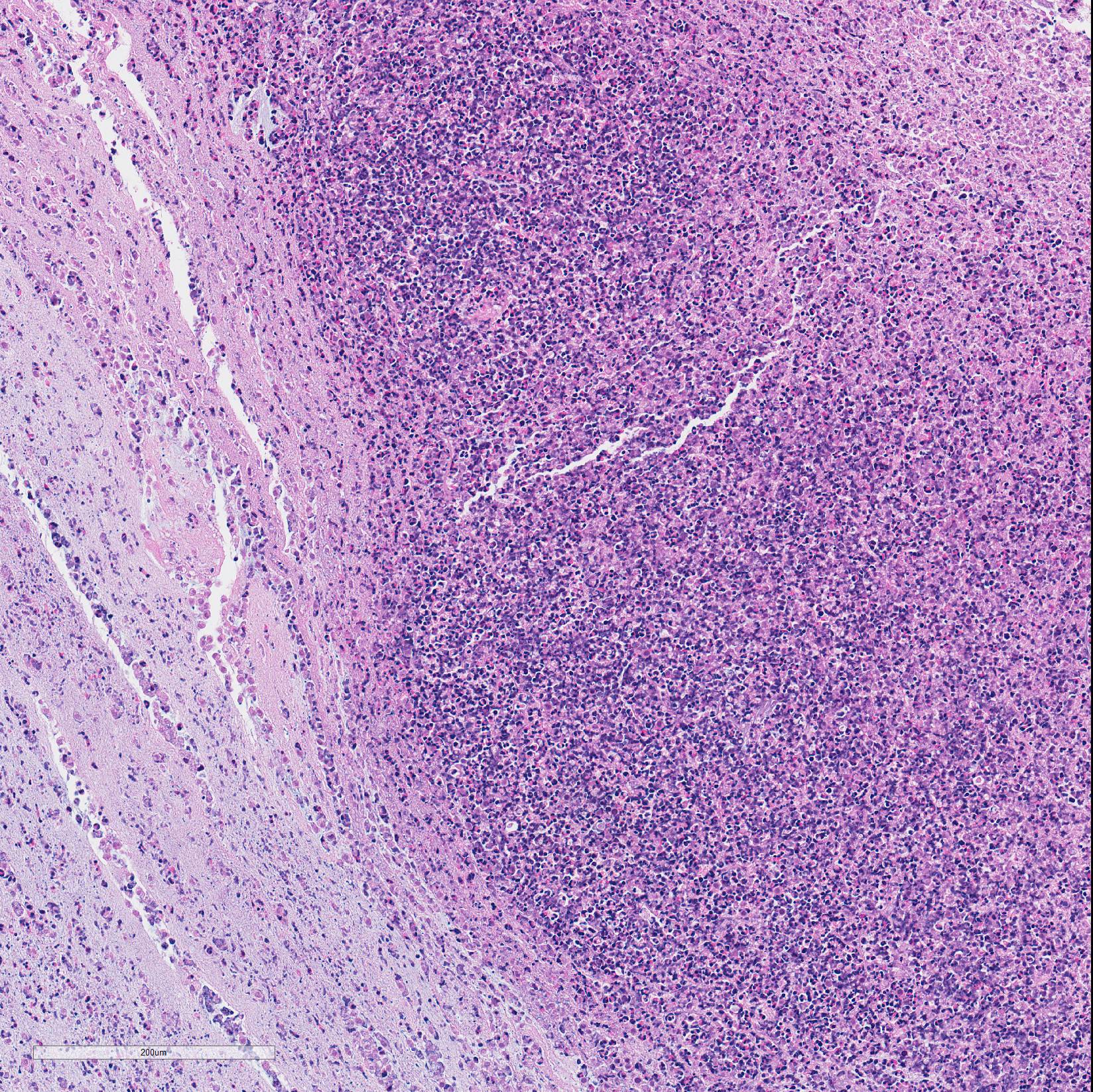
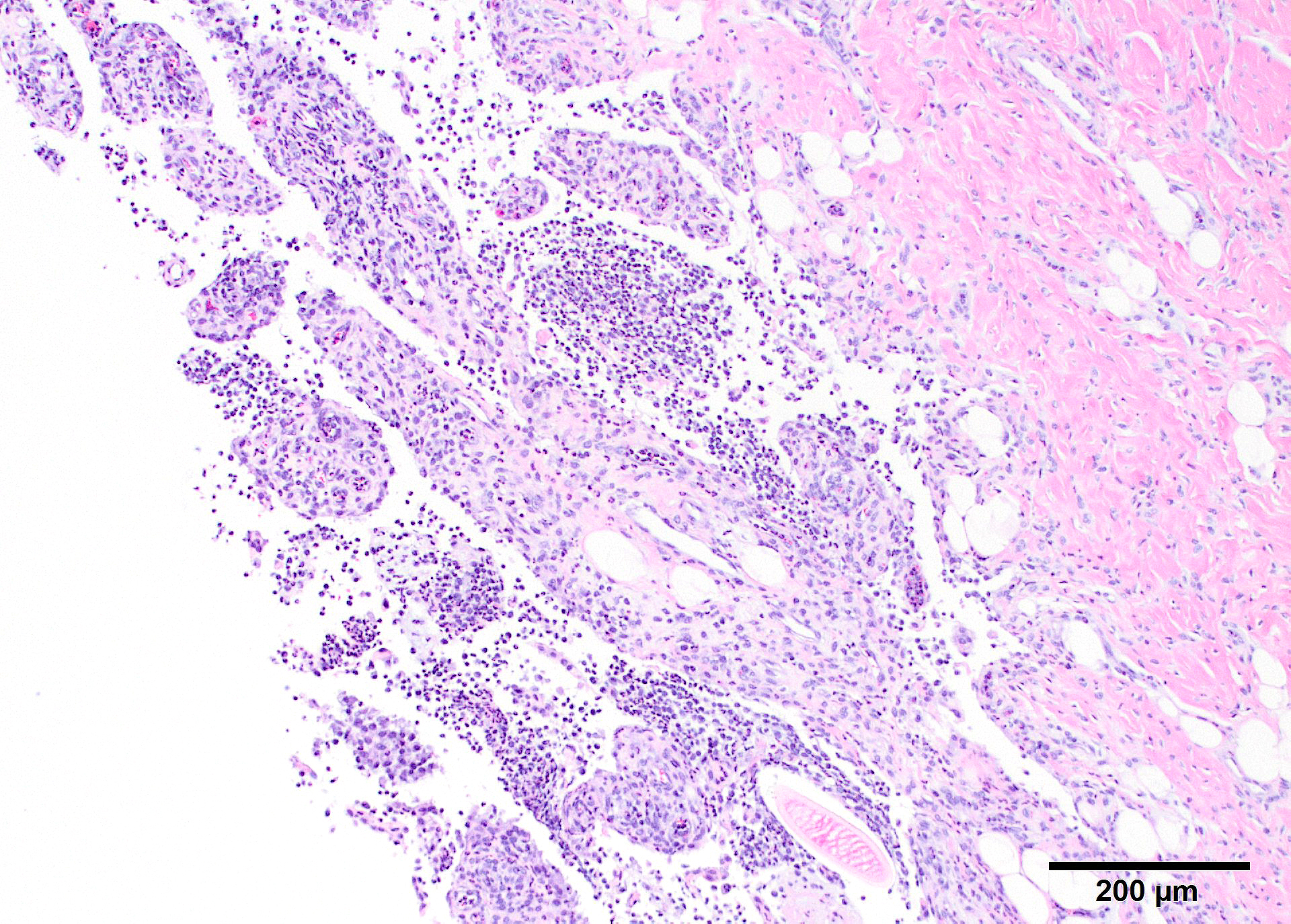
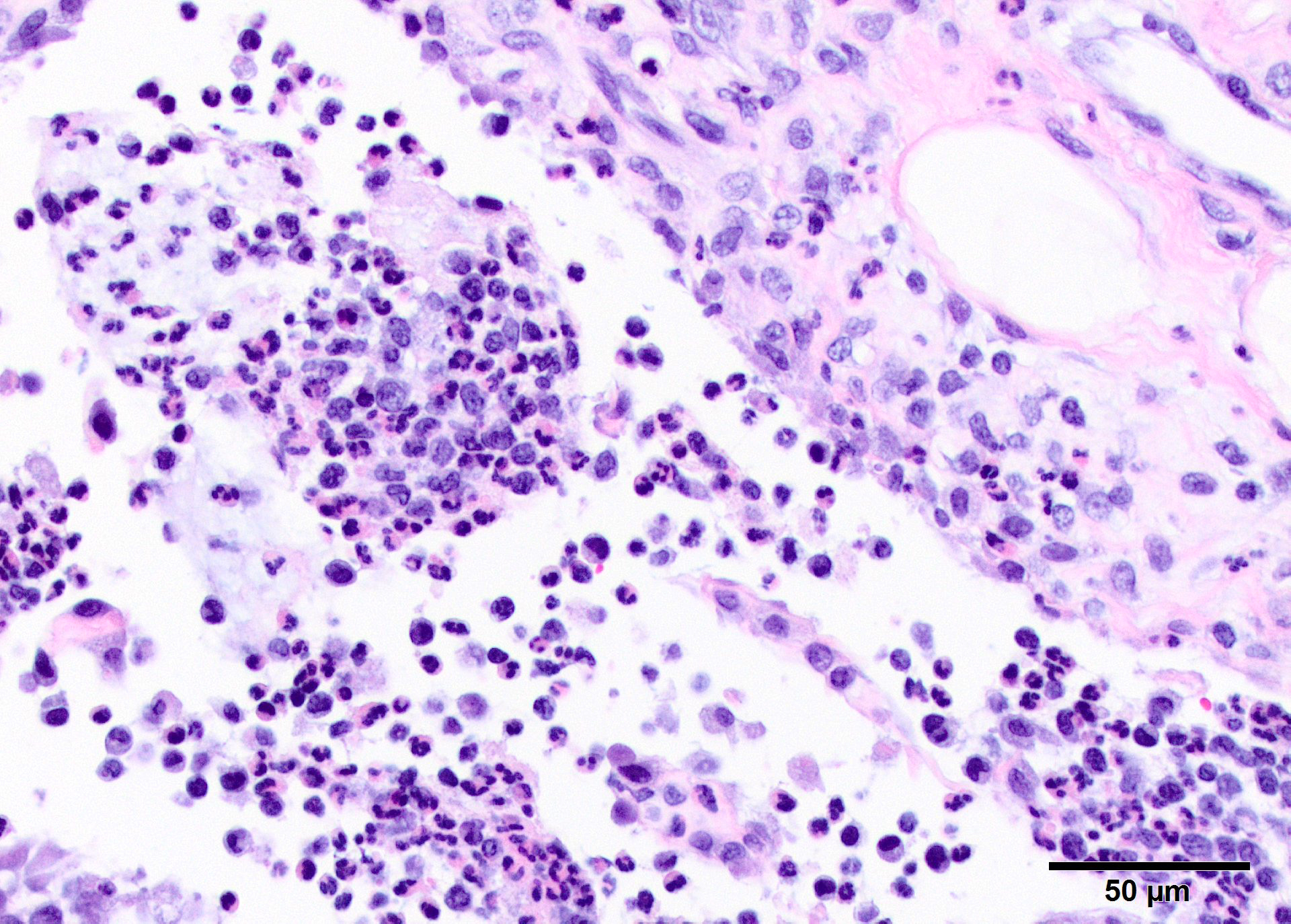
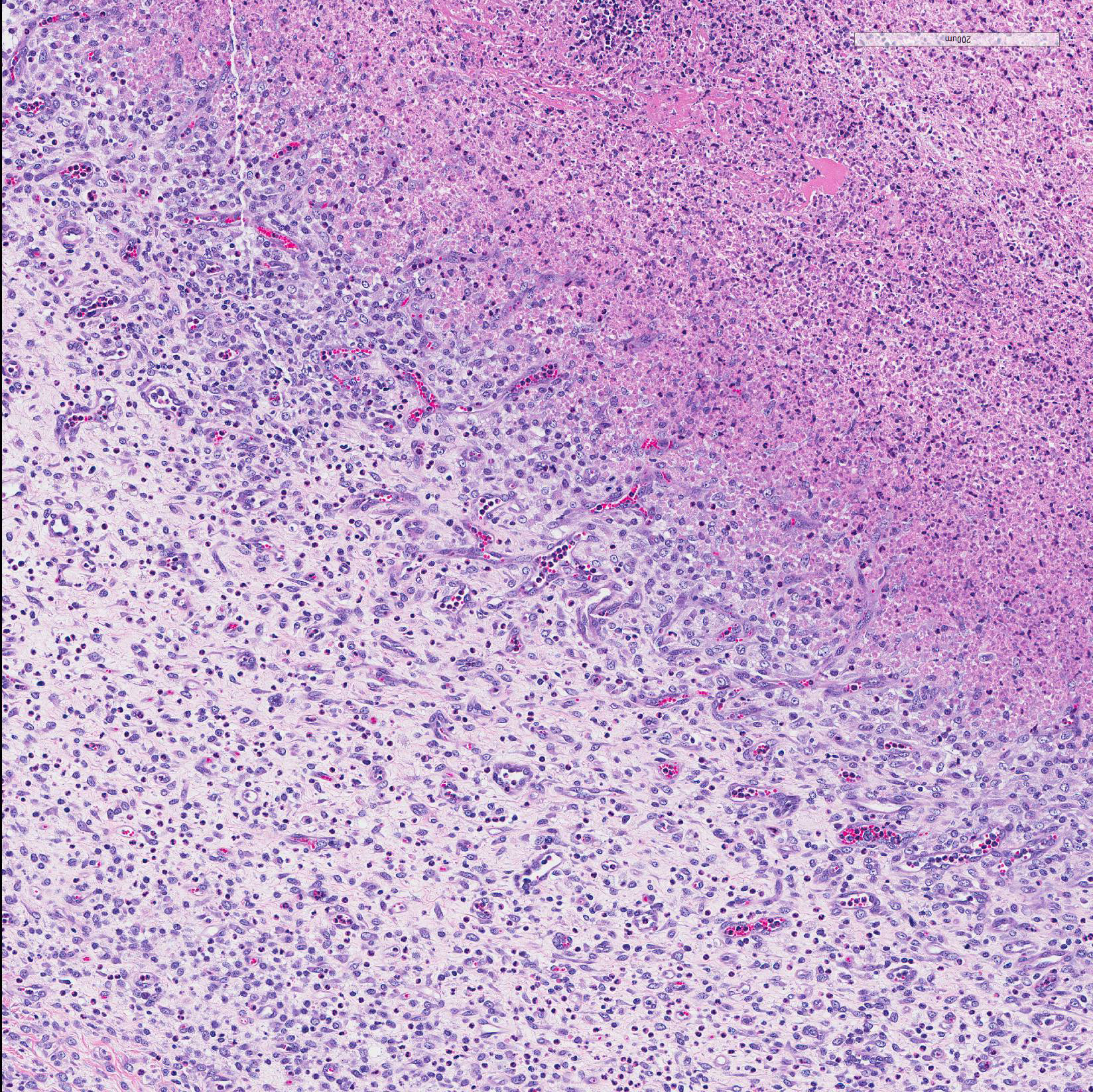
Microscopic Description: In all joints examined, there was marked edema and inflammation of the synovial membrane. The least affected specimen still contains recognizable synovial villi (Fig 2). Neutrophilic inflammation occurred in the joint lumen and infiltrating villi (Fig 3), admixed with other leukocytes. The synovial lining was ulcerated in other joints, with more fibrin in the joint lumen. The synovium and joint capsules were expanded by edema, moderate fibrosis and granulation tissue. Multifocally within the synovium, capsule, joint space, ligament, and adjacent fat and muscle, there were aggregates of necrotic neutrophils with fewer macrophages, similar to those comprising the separated inflammation in the specimens submitted. Unfortunately in these particular specimens, it is only possible to identify the sheets of necrotic neutrophils, not their location. The extent of inflammation is illustrated in Fig 4, taken from another affected joint.
Contributor?s Morphologic Diagnosis:
Subacute fibrinosuppurative and necrotizing polyarthritis
Contributor?s Comment: The synovial lesions found in this goat are characteristic of Mycoplasma mycoides subsp. capri infection, with regard to the extent and severity of inflammation. Clumps of degenerate neutrophils and extension of inflammatory cells across the joint capsule and occasionally into muscle are dissimilar to arthritis of neonatal bacteremia, or early lentiviral infection. Several species of mycoplasma, including M.capricolum subsp. (the etiology of contagious caprine pleuropneumonia), M. putrifaciens and M agalactia can produce similar arthritis.9,11 This particular goat had fibrinous pleuritis as well, and pleuropneumonia is often described as the most common pathologic finding in herd outbreaks, which can produce explosive disease and high morbidity and mortality. In contrast, arthritis associated with neonatal bacteremia yields a more localized suppurative intra-articular exudate with less extensive synovitis. Lentiviral infection leads to synovial proliferation and a lymphohistiocyic exudate.
Mycoplasma mycoides subsp. capri has been described in herd outbreaks involving hundreds of goats,3,5,12 and, although this outbreak involved only kids less than a month of age, adults can also be affected. Adults can carry the organism in the ear canal, and isolation or PCR are the main methods of detecting carriers to eliminate them from the herd.1 Kids acquire the infection by ingesting the agent through milk or colostrum containing mycoplasma.5 Experimental infection after systemic innoculation8 recapitulates the disease in multiple organ systems.
It is important to recognize that organisms of the mycoides group of mycoplasmas is somewhat distantly related from most other species6,4 and that PCR tests that can speciate this group of organisms must be used or the organism may escape detecton.2,7,10
Contributing Institution:
Norwegian University of Life Sciences, Faculty of Veterinary Medicine www.nmbu.no
JPC Diagnosis: Disarticulated joint: Synovitis, fibrinosuppurative, chronic, diffuse, severe, with synovial ulceration and marked granulation tissue formation.
JPC Comment: Outbreaks of disease in young goats due to Mycoplasma mycoides subsp. capri have been reported to cause serious disease around the world for many years. In addition to polyarthritis, the agent may also result in septicemia as well as respiratory disease in kids. The disease is introduced into a herd by healthy carriers, and as mentioned by the contributor, transmitted to young kids via infected milk (up to 50% of does shedding mycoplasma in the milk do not show clinical signs.).1 The feeding of pooled milk by bottle appears to be the most common route of infection. Disease outbreaks often occur following stress, such as the onset of cold weather or overcrowding. Infected animals may be successfully treated with tylosin, and closer attention fo husbandry practices as well as a long period allowd for kids to nurse from their dams appears to be useful in preventing recurrence of the disease in out years.
Mycoplasmia agalactiae, the causative agent of contagious agalactiae in
goat, may also cause arthritis in neonatal goats.5 An outbreak in
Greece demonstrated polyarthritis in 1-3 day old kids the season after the doe
herd had contracted contagious agalactia, and the dams of the affected kids had
been treated during that time for mastitis. (Affected dairy goats usually shed
mycoplasma in the milk for a year, although prolonged shedding for periods up
to 8 years have been reported.)5 Mycoplasma mycoides subsp. mycoides
has also been reported to cause polyarthritis in goats as well.5
Since the submission of this case, the contributors have published a case study in the Journal of Veterinary Diagnostic Information. Over a three year period 8 goats kids, averaging 2wks of age were submitted for autopsy. 5 of the 8 goats had concurrent respiratory disease (pleuropnemonia, interstitial pneumonia, or pleuritic) 2 had meningitis, and 1 (presumptive the individual in this case) had pericarditis as well. PCR testing of colonies grown on sheep blood trypticase soy agar from affected animals using universal 16S rRNA forward and reverse primers identified the isolates as M. mycoides subsp capri
References:
1. Agnello S, Chetta M, Vicari D, Mancuso R, Manno C, Puleio R, Console A, Nicholas RAJ, Loria GR. Severe outbreaks of polyarthritis in kids caused by Mycoplasma mycoides subspecies capiri in Sicily. Vet Rec 2012; 170,416b doi: 10.1136/vr.100481
2. Amores J, et al. Comparison of culture and PCR to detect Mycoplasma agalactia and Mycoplasma mycoides subsp. Capri in ear swabs taken from goats. Vet Microbiol. 2010;140:105-108.
3. Becker CAM, Ramos F, Sillal E, et al. Development of a multiplex real-time PCR for contagious agalactia diagnosis in small ruminants. J Microbiol Meth. 2012;90:73-79.
4. DaMassa AJ, Eakenell PS, Brooks DL. Mycoplasmas of goats and sheep. J Vet Diagn Invest. 1992;4:101-113.
5. Filioussis G, Giadinis ND, Petridou EJ, Karavanis , Papageorgios K, Karatzias H. Congenital polyarthritis in goat kids attributed to Mycoplasma agalactiae. Vet Rec 2011; 169,364b doi: 10.1136/vrd627
6. Fischer A, Shapiro B, Muriuki C, et al. The origin of the ?Mycoplasma mycoides? cluster coincides with domestication of ruminants. PLOS ONE 2012;7:e36150.
7. Johnson GC, Fales WH, Shoemake BM, Adkins PR, Middleton JR, Williams III F, Zinn M, Michell WJ, Calcutt MJ. An outbreak of Mycoplasma mycoides subspecies capri arthritis in young goats: a case study. J Vet Diagn Invest 2019; 31(3):453-457.
8. Kinde H, DaMasse AJ, Wakenell PS, et al. Mycoplasma infection in a commercial goat dairy caused by Mycoplasma agalactia and Mycoplasma mycoides subsp. mycoides (caprine biotype). J Vet Diagn Invest 1994;6:423-427.
9. Liu W, Fang L, Li M, et al. Comparative genomics of Mycoplasma: analysis of conserved essential genes and diversity of pan-genome. PLOS ONE 2012;7:e35698.
10. McAuliffe L, Ellis RJ, Lawes JR, ,et al. 16S rDNA PCR and denaturing gradient gel electrophoresis; a single generic test detecting and differentiating Mycoplasma species. J Med Microbiol. 2005;54:731-739.
11. Nakagawa M, Taylor WD, Yedloutschnig RJ. Pathology of goats and sheep experimentally infected with Mycoplasma mycoides var. capri. Nat Inst Hlth Quart.1976:16:65-77.
12. Nicholas RAJ. Improvements in the diagnosis and control of diseases of small ruminants caused by mycoplasmas. Small Rum Res. 2002;45:145-149.
13. Richter DJ, Rurangirwa FJ, Call DR, et al; Development of a bead-based multiplex PCR assay for the simultaneous detection of multiple Mycoplasma species Vet Microbiol. 2011;153:246-256