Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 25
6 May, 2020
CASE III: 17-137-W4 (JPC 4117671).
Signalment: ~6 month old, female, Yorkshire cross, Sus scrofa
History: Animal arrived at the university on 10/3/17. Observed limping on right front limp on 10/6/17, three days after arrival. The lameness progressed despite NSAID and antibiotic administration. The animal was also observed coughing occasionally with mucous production. Radiographs indicated soft tissue swelling and evidence of pneumonia. The decision was made to euthanize by the investigator on 10/12/17 due to lack of response to treatments and inability to use in the following week's study. A complete necropsy was performed by the veterinary pathologist on 10/13/17.
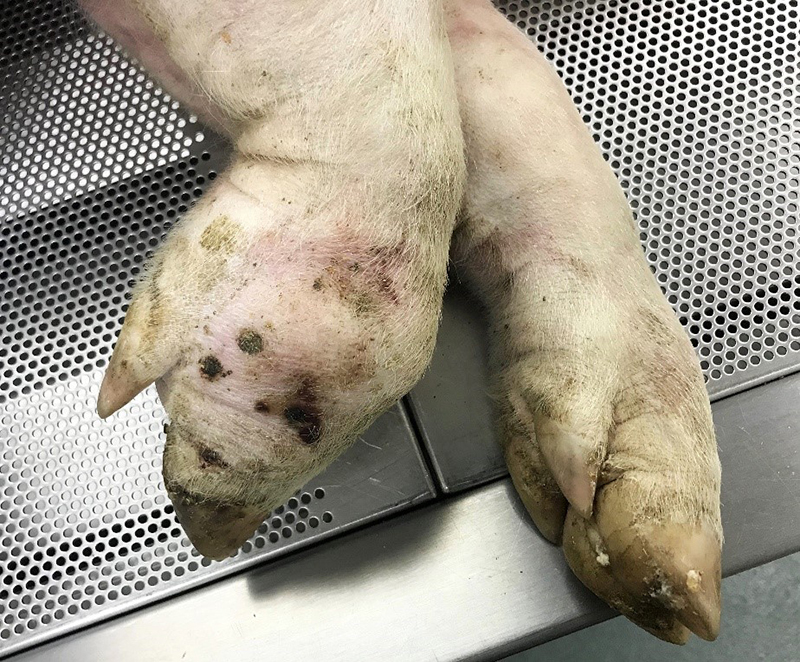
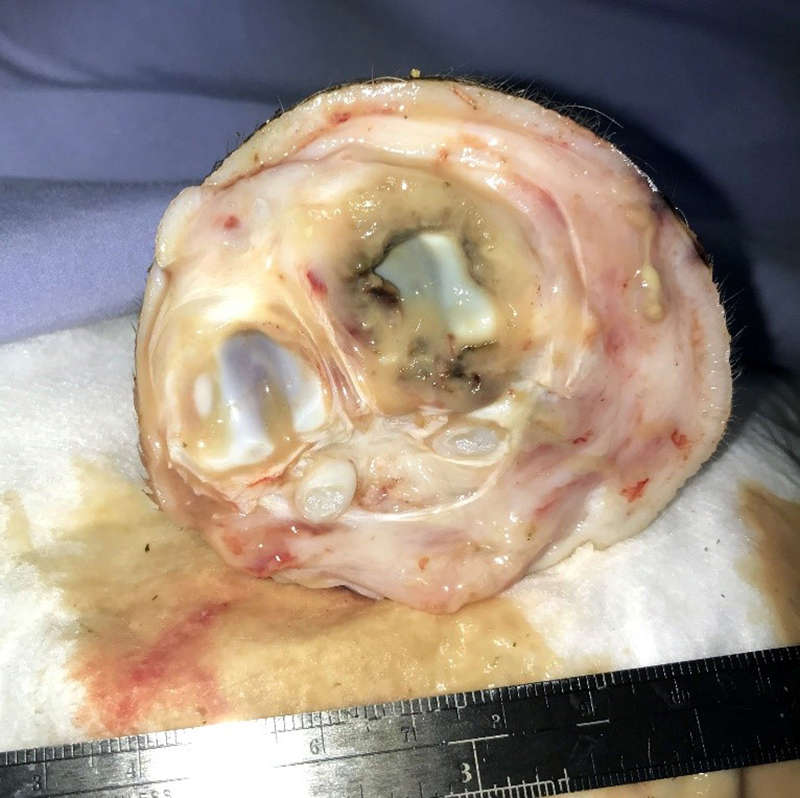
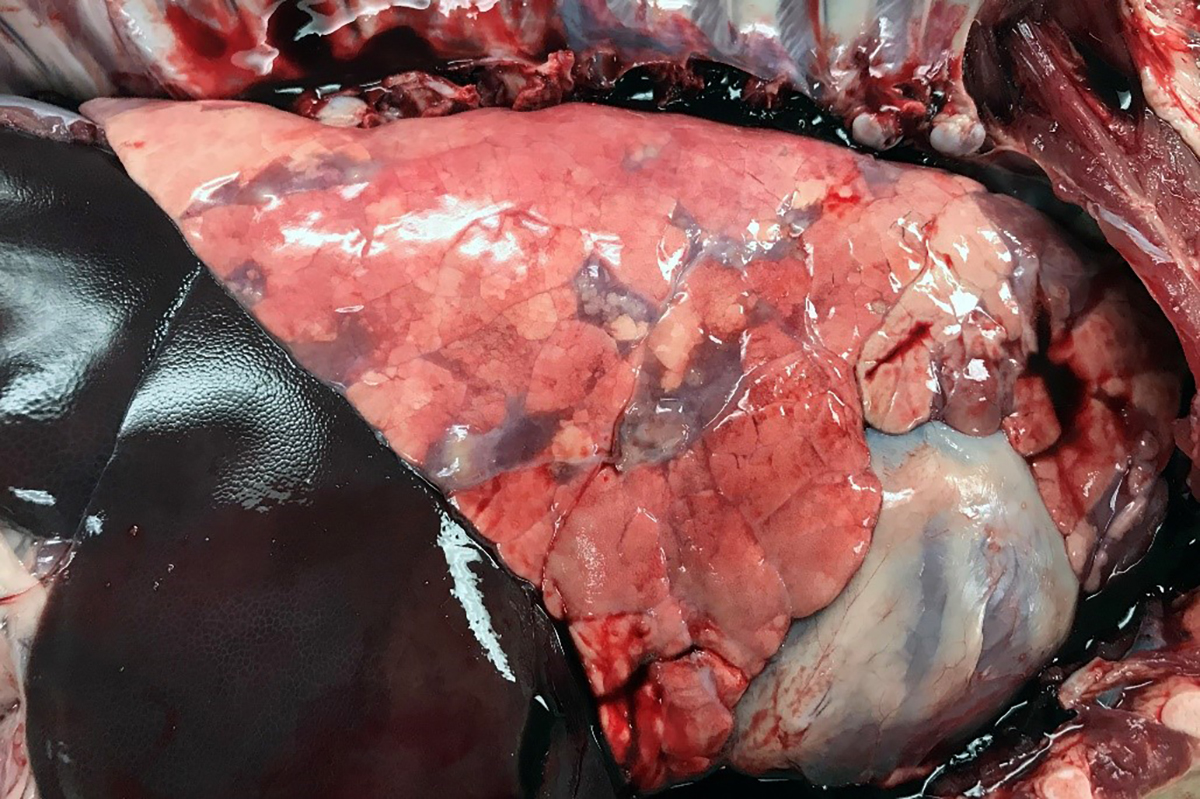
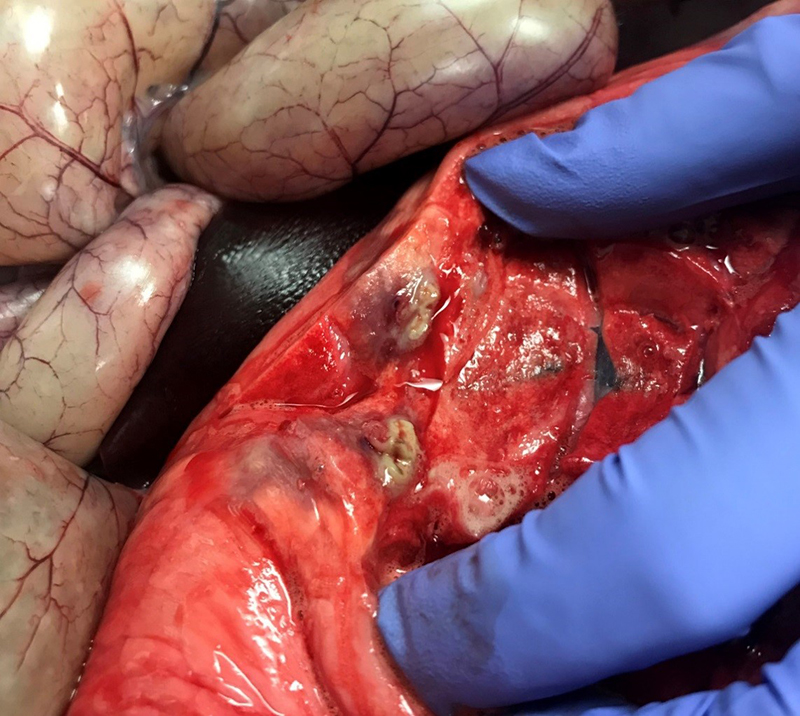
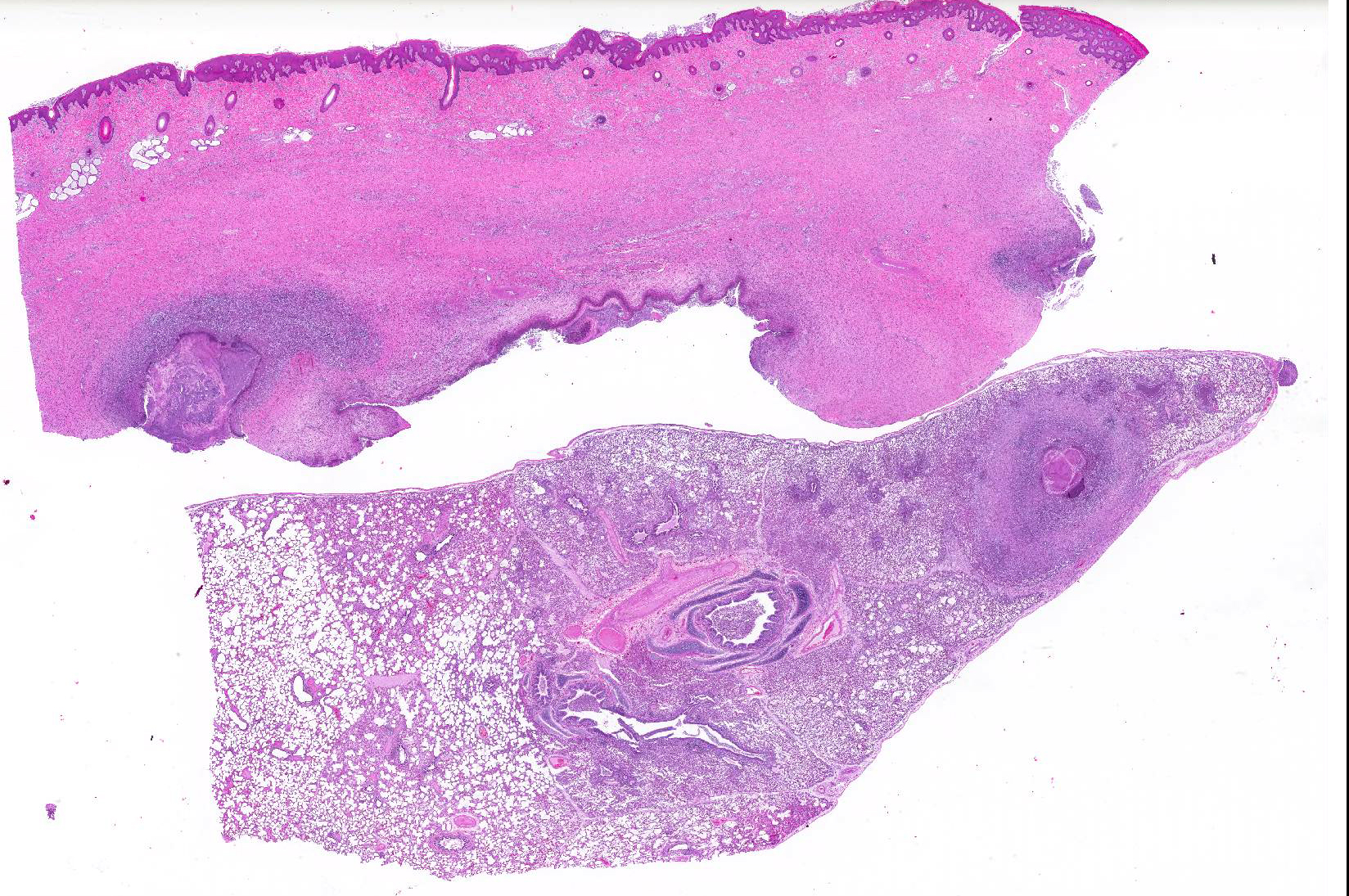
Gross Pathology: The right front foot swelling was associated with a large joint abscess between P2 and P3. Approximately 20mL of tan foul smelling fluid was expressed when the joint capsule was cut. The synovium was proliferative and tan-grey to dark red between the lateral P2 - P3 joint. There was also circumferential severe swelling of the surrounding soft tissues. The lungs contained multifocal lobular dark red firm areas. On cut section, lobules were dark red to grey with white-grey foci that seemed to be associated with the airways. Cultures of the lung were submitted.
Laboratory results: Cultures from both the lungs and joint were positive for the bacterial organism Trueperella pyogenes.
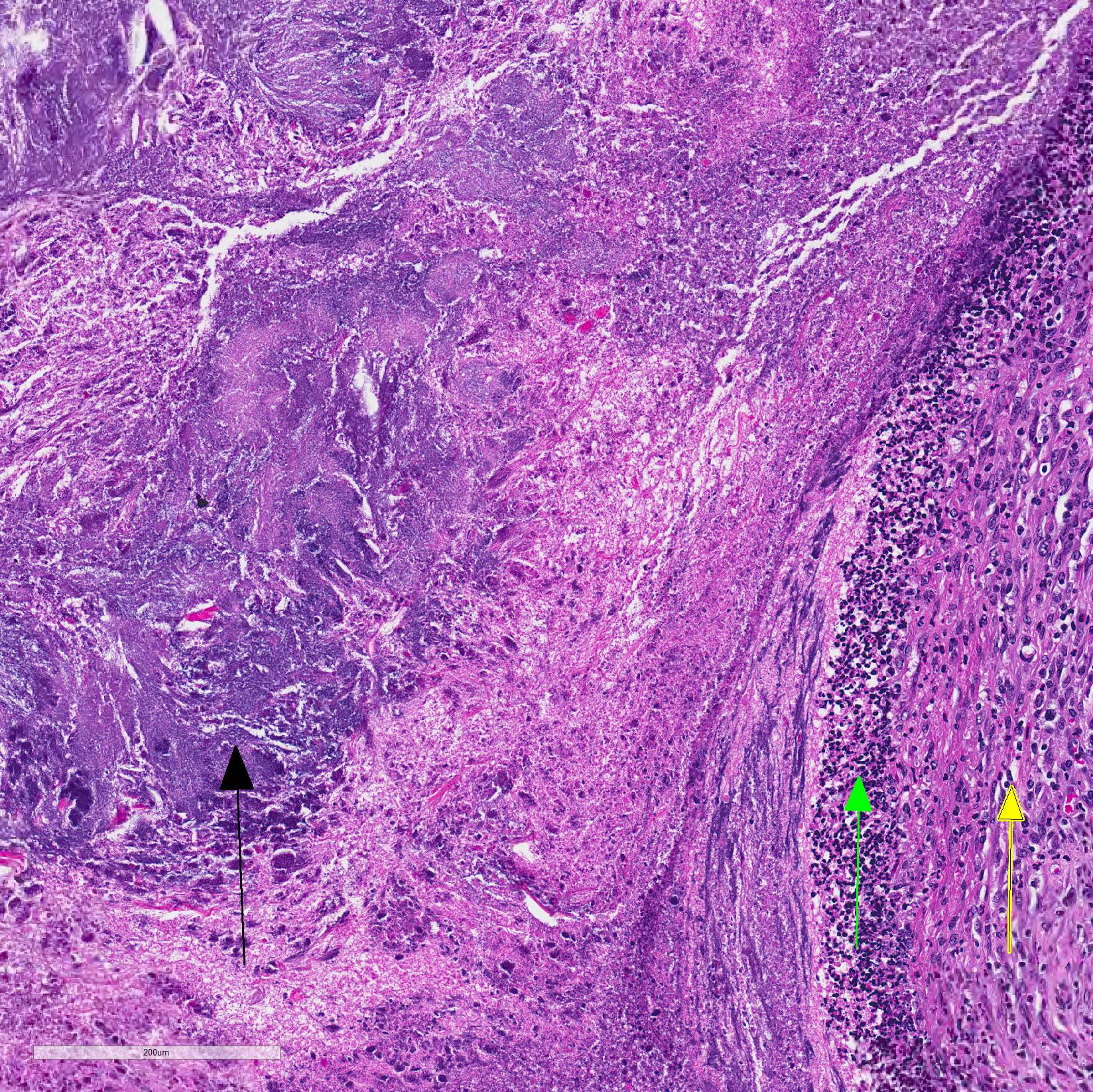
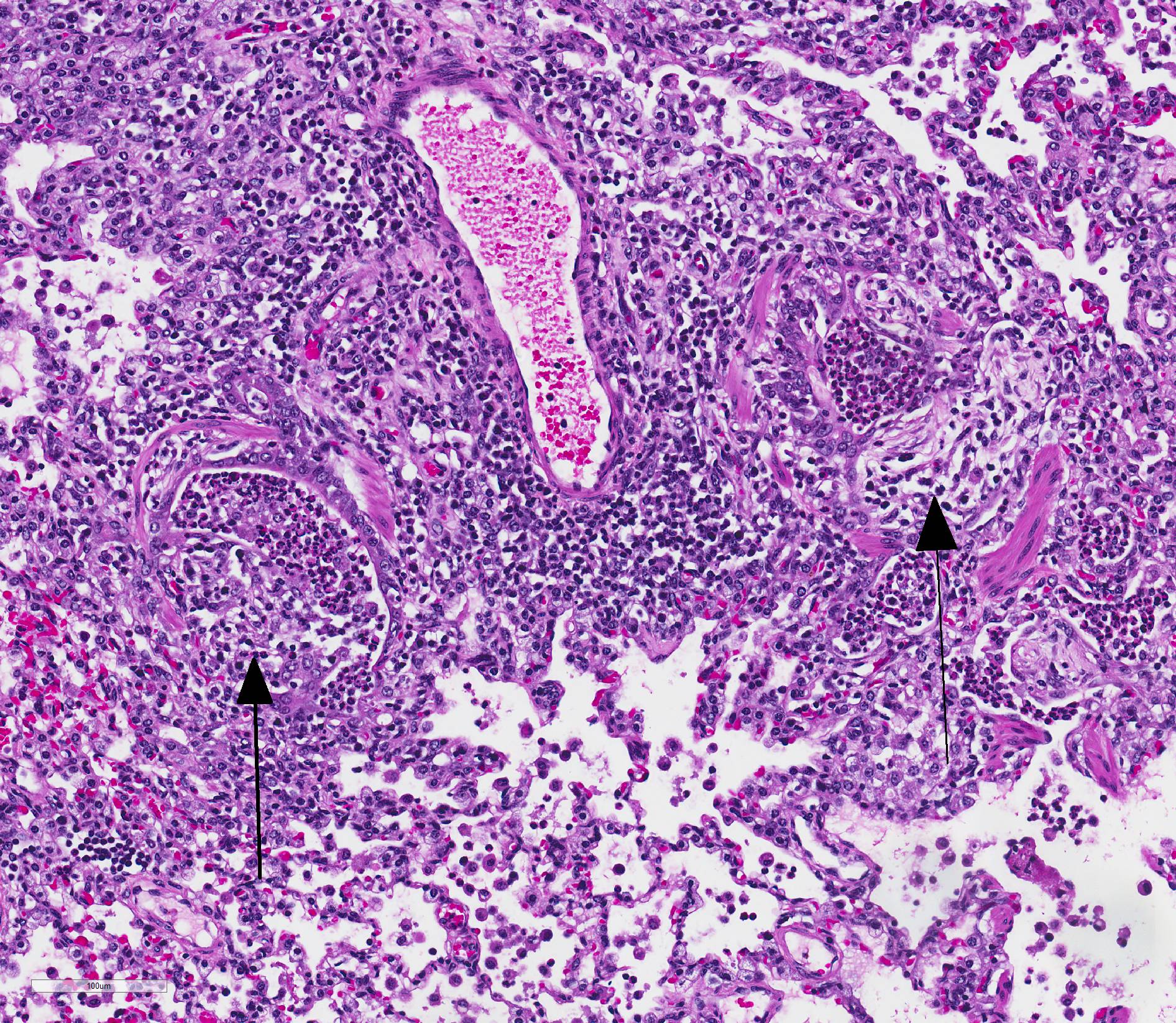
Microscopic Description: Lungs: Affecting 75% of the section, there are multifocal to coalescing areas of necrosis composed of numerous viable and degenerate neutrophils with fewer macrophages admixed with abundant karyorrhectic and eosinophilic cellular debris and cloud-like bacterial colonies. Necrotic foci are surrounded by many epithelioid macrophages, reactive fibroblasts, and fewer lymphocytes with an outside rim of organizing granulation tissue and collagen. In less affected areas, the perivascular interstitium and alveolar septa are infiltrated and expanded by variable numbers of macrophages, neutrophils, and lymphocytes. Alveoli are often filled with proteinaceous fluid (edema), fibrin, foamy macrophages, and neutrophils. Most airways contain various amounts of neutrophils, fibrin, edema fluid, and sometimes bacterial organisms. Numerous blood vessels in the lung sections contain fibrin thrombi.
Joint, P2-P3: The synovial lining is diffusely ulcerated and covered in a layer of hyalinized fibrin and necrosuppurative debris. The joint capsule contains microabscesses composed of a central necrotic core surrounded by a rim of epithelioid macrophages, more peripheral reactive fibroblasts and lymphocytes, with an outside layer of organizing granulation tissue and collagen. The synovial membrane is thickened up to 5 times normal and forms villous projections in areas. Villi are expanded by edema, fibrin, congested blood vessels, fibroblasts, organizing granulation tissue and fibrous connective tissue.
Contributor Morphologic Diagnosis:
Pneumonia, necrosuppurative, multifocal to coalescing, severe, chronic, with bacteria, etiology consistent with Truperella pyogenes.
P2-P3 synovitis, necroulcerative, diffuse, chronic, with microabscesses and bacteria, etiology consistent with Truperella pyogenes
Contributor Comment: The lesions in this animal indicated chronic infection in both the joint space (P2-P3) and the lung was due to Truperella which was cultured from both locations. Due to the severity and chronicity of the lesions, it is not possible to determine whether they occurred as a result of direct infection (i.e.: inhalation, focal wound) or embolic spread. The large amount of airway inflammation in the lungs suggests possible infection by inhalation, but embolic spread was likely also a factor.
T. pyogenes is a commensal and an opportunistic pathogen that causes abscessation and pneumonia in several large animal species including pigs and ruminants. In addition to pneumonia and abscesses, other potential lesions include metritis, udder lesions, pneumonia, arthritis, endocarditis, lymphadenitis and osteomyelitis. It is a gram-positive, non-motile, non-spore-forming, short, rod-shaped, coryneform bacterium that is ubiquitous in the environment and can be found on the mucous membranes of domestic animals leading to subsequent spread to any other organ system in the body.2,3,4,7
Although T. pyogenes is can infect companion animals and humans, it is an uncommon clinical pathogen.1 In ruminants and pigs, however, it can cause significant disease and lead to condemnation at slaughter. Although, treatment with antibiotics can clear the infections, this pathogen can also develop resistance to commonly used antibiotics such as tetracycline and doxycycline.2,4,7
Contributing Institution:
University of Pittsburgh, Division of Laboratory Animal Resources
http://www.dlar.pitt.edu/
JPC Diagnosis: 1.
Haired skin, deep dermis: Abscess, with draining tract.
2. Lung: Pneumonia, embolic, chronic-suppurative, focally extensive, severe,
with bronchiolitis obiliterans.
JPC Comment: Well-known
for its opportunistic infections in ruminants, Trueperella pyogenes is a common
cause of suppurative lesions in swine as well. T. pyogenes is a common
inhabitant of the mucosal flora in healthy animals, and can be cultured from
feces of healthy swine. Infected swine can shed it in urine, feces and
discharges from the upper respiratory tract, teats, and vulva.6
As an opportunist, it colonizes areas of inflammation or infection caused by
other agents, and is a common isolate in longstanding lesions such as abscess
when the inciting agent has long disappeared. The bacterium possess a number
of virulence factors which help in colonization of a number of surfaces,
including neuraminidases, fimbria and collagen-, fibrin-, and fibrinogen binding
proteins. Other virulence factors assist in tissue damage, including
pyolysin, a cholesterol-binding exotoxin which results in cytotoxicity in a
variety of cells, including neutrophils, macrophages, epithelial cells,
erythrocytes, and fibroblasts.5 The cytolytic activity of pyrolysin
is similar to other exotoxins secreted by gram-positive bacteria, by binding to
the cell membrane and forming transmembrane pores. 5
T. pyogenes is one, if not the most common bacterium isolated with suppurative processes in swine, and it may be responsible not only for focal lesions, but as a result of hematogenous dissemination (as demonstrated in this case), multisystemic infections as well. It is a common agent of pneumonia, pleuritic, osteoarthritis, polyarthritis, mastitis, endocarditis, valvulitis, and reproductive infections.5,6 It may result in pyelonephritis or mastitis in non-pregnant sows, and pyometra with fetal maceration in pregnant sows. It may also be part of a mixed infection, and infections are often associated with immunosuppression from common swine viruses, such as porcine arterivirus (PRRSV).5
Abscesses in a variety of organs are characterized by a thick wall and yellow-green pus. Intramuscular or subcutaneous abscesses may show few clinical signs except a loss of body condition in pigs, but will often result in carcass condemnation at slaughter, especially in cases with multiple abscesses or visceral involvement.5
References:
1. Billington SJ, Post KW, Jost BH. Isolation of Arcanobacterium (Actinomyces) pyogenes from cases of feline otitis externa and canine cystitis. J Vet Diagn Invest. 2002; 14:159-162.
2. Buddle JR, O?Hara AJ. Enzootic pneumonia of pigs a diagnostic dilemma. Aust Vet J. 2005;83:134_139.
3. Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Vol. 2. 6th ed. London, UK: Saunders Elsevier; 2016. Volume 1:148
4. Ribeiro MG, Risseti RM, Bolaños CAD, Caffaro KA, de Morais ACB,
Lara GHB, Zamprogna TO, Paes AC,
Listoni FJP, Franco MMJ. Trueperella pyogenes multispecies infections in
domestic animals: a retrospective study of 144 cases (2002 to 2012). Veterinary
Quarterly. 2015; 35(2): 82-87.
5. Rzewuska
M, Kwiecien E, Chrobak-Chmiel D, Kizerwetter-Swida M, StefanskaI, Gierynska M.
Pathogenicity and virulence of Truperella pyogenes: a review. Int J Molec
Sci 2019; 20(11). pii: E2737. doi:
10.3390/ijms20112737.
6. Taylor DJ. Misclennaeous bacterial infections. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW eds., Diseases of Swine 10th ed. Ames IA, John J. Wiley & Sons, pp. 867-868.
7. Zhang D, Zhao J, Wang Q, Liu Y, Tian C, Zhao Y, Yu L, Liu, M. Trueperella pyogenes isolated from dairy cows with endometritis in Inner Mongolia, China: Tetracycline susceptibility and tetracycline-resistance gene distribution. Microbial Pathogenesis. 2017:51-56