WSC 2023-2024, Conference 22, Case 1
Signalment:
18-month-old Angus heifer, Bos taurus, bovine.
History:
Two out of a herd of one hundred heifers had a history of progressive wasting and diarrhea. One of the heifers had a body condition score of 1/5 and was euthanised.
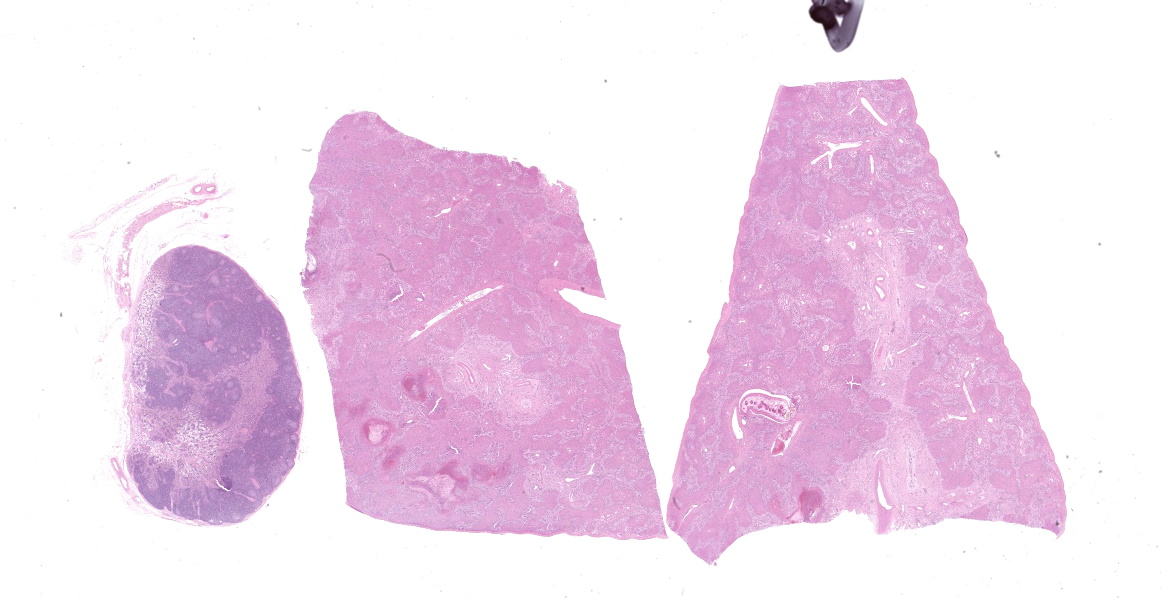
Gross Pathology:
A field post-mortem was performed and over 10 adult liver flukes were observed in the liver parenchyma, as well as occasional multifocal areas of chronic pulmonary consolidation and suspected ileum thickening.
Laboratory Results:
Serum liver fluke ELISA S/P % - 158 (strong positive).
Serum pestivirus antigen capture ELISA - negative.
Bovine Johne’s disease liquid culture and PCR - negative.
Microscopic Description:
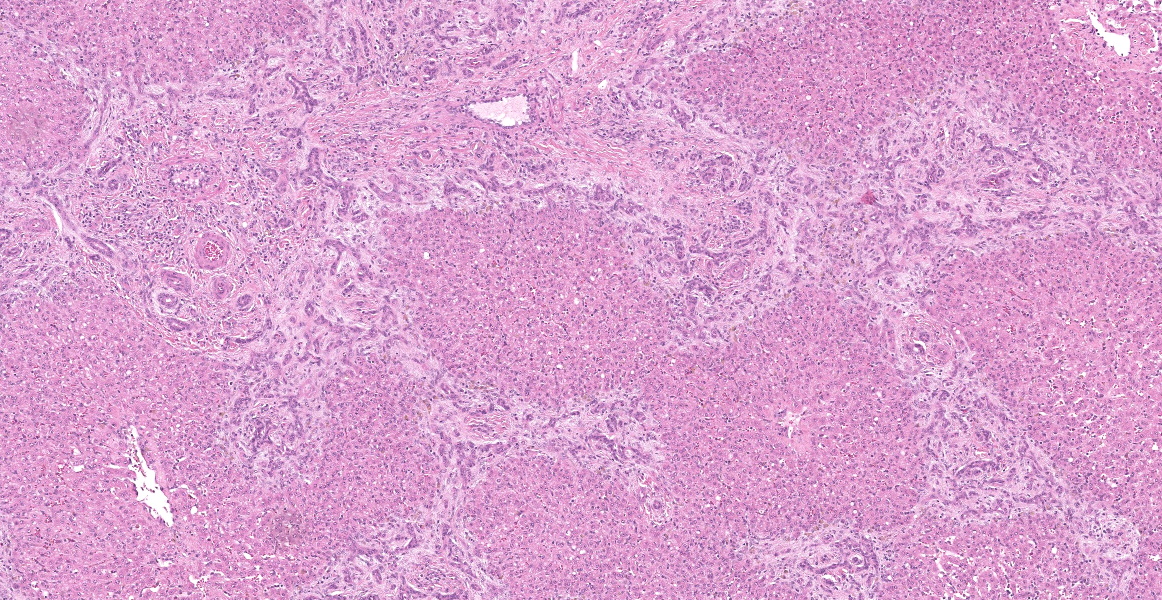
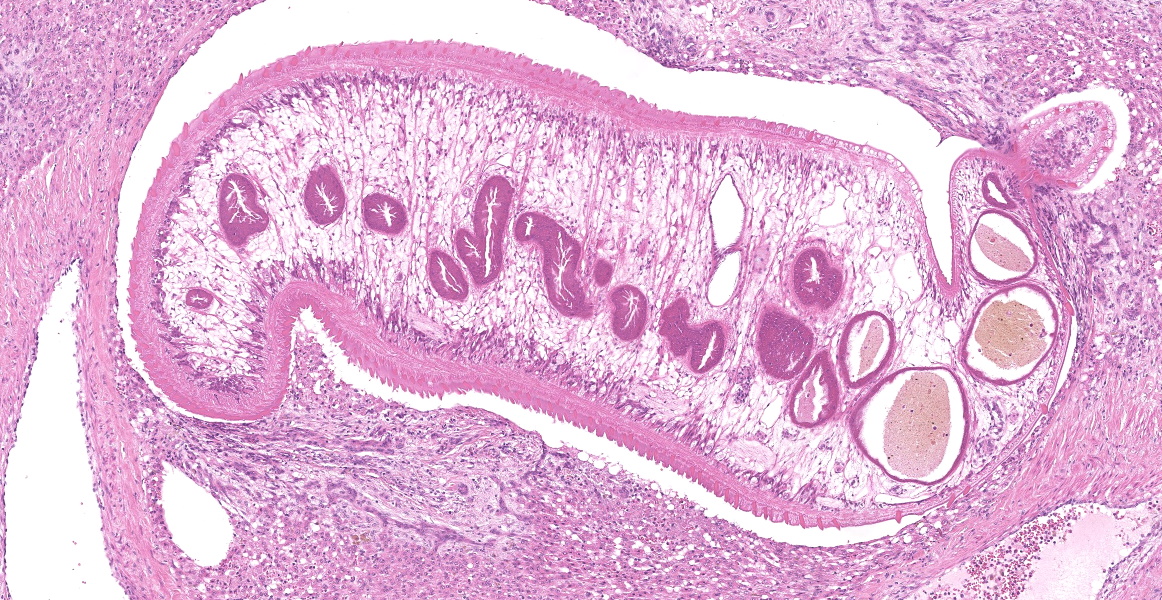
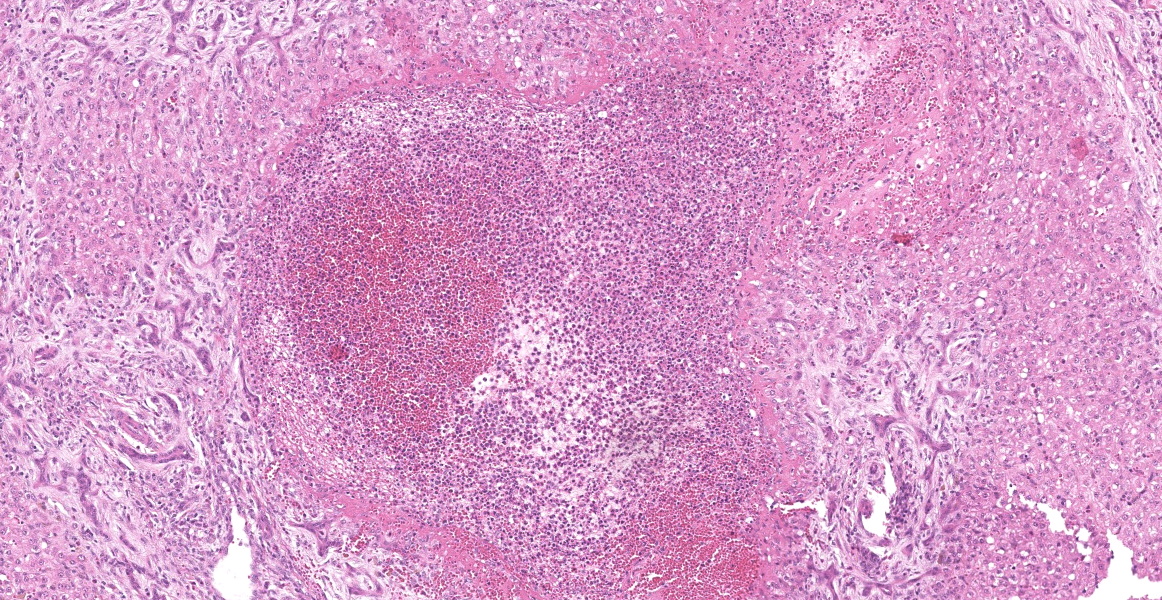
Portal areas are markedly expanded by proliferative fibroblasts, mature connective tissue, markedly increased numbers of small caliber bile ducts (biliary hyperplasia) replacing periportal hepatocytes and frequently joining between adjacent portal areas and isolating hepatic lobules (bridging fibrosis), and numerous eosinophils and lymphocytes. Macrophages scattered in portal areas and sinusoids often contain yellow-brown pigment (likely bile). Multifocally replacing parenchyma, there are several irregular, well demarcated areas of homogenous eosinophilic material (proteinaceous oedema) with extravasated erythrocytes (haemorrhage) and frequent intact and degenerate eosinophils and macrophages, surrounded by fibrillar eosinophilic material (fibrin) (liver fluke migration tract). Focally replacing the parenchyma, there is a longitudinal section of a 3.1 x 0.9 mm metazoan parasite which features a prominent outer tegument with spines on the anterior two thirds, muscular anterior (oral) and ventral suckers, with a digestive tract which includes a caecum containing yellow-brown pigment, surrounded by parenchyma, and no evidence of a uterus, vitellarian glands, or eggs (morphology consistent with juvenile Fasciola hepatica).
Contributor’s Morphologic Diagnosis:
Liver: Fibrosis, periportal and bridging, marked, diffuse, chronic, with marked biliary hyperplasia, eosinophilic and lymphocytic portal hepatitis, parasitic migration tracts and intralesional juvenile trematode, morphology consistent with Fasciola hepatica, Bos taurus.
Contributor’s Comment:
Liver fluke (Fasciola hepatica) is a common endoparasite of cattle and sheep which is endemic to Eastern Australia and poses a major economic threat to producers.1 The life cycle of the liver fluke in the animal begins with excystment of metacercariae in the small intestine where newly emerged juvenile flukes migrate to the liver and then over 8-10 weeks migrate through the hepatic parenchyma to the bile ducts. Here, they mature into adult flukes and produce eggs in the bile and feces 8-10 weeks post infection.2 In the acute phase, the migration through the liver causes extensive inflammation and hepatocellular damage, which induces an antibody response.2
There are several different methods used to diagnose liver fluke, including fecal sedimentation examination, necropsy, histopathology, and immunological based tests which include an antibody ELISA, or a commercial fecal copro-antigen ELISA (cELISA; Bio X diagnostics, Belgium). Each method has advantages and disadvantages, and test results should be interpreted in light of the tests’ shortcomings.
Fecal sedimentation results in cattle have variable sensitivity (30-60%) due to the intermittent shedding of the fluke eggs, volume of the feces produced, and the relatively long prepatent period.14 The specificity is high at 99%.7 This test will only detect the presence of female adult fluke.
Liver fluke antibody ELISA may be performed on serum and milk and will detect IgG antibodies for F. hepatica excretory- secretory (E/S) antigen from 2-4 weeks post infection.13 The sensitivity and specificity for the serum antibody ELISA varies depending on the season and ranges from 72-94% and 76-89%, respectively.7 Another advantage of the antibody ELISA is that it can be used for surveillance and herd level diagnosis in bulk milk samples with ranges of sensitivity and specificity being 81.3-95% and 98.2-100%, respectively.11 The antibody ELISA can only detect exposure and may remain positive for at least 4 weeks post flukicide treatment; therefore, veterinarians should take caution interpreting these results as cattle may not be currently infected.2
|
Test |
Sensitivity (%) |
Specificity (%) |
|
Fecal sedimentation |
30-6012 |
9912 |
|
Liver fluke serum antibody ELISA |
72-946 |
76-896 |
|
Liver fluke milk antibody ELISA |
81-959 |
98-1009 |
|
Commercial fecal copro-antigen ELISA |
80-878 |
1008 |
|
Liver necropsy |
996 |
986 |
|
Abattoir liver inspections |
686 |
886 |
|
Table 1: Summary of sensitivity and specificity for different diagnostic tests for F. hepatica detection.7 |
||
The cELISA detects F. hepatica E/S antigen in the feces 6-8 weeks post-infection and can detect low liver fluke burdens.6 The cELISA was designed for and is commercialized for the use of detecting liver fluke in sheep. Many studies have reviewed the use of the fecal copro-antigen ELISA in cattle. Palmer et al. (2014) illustrated that the specificity of the cELISA was 100% in cattle.9
When following the recommended cut off for the commercial kit the sensitivity was 80% in comparison to 87% when using a lower custom cut off for liver fluke detection in Australian cattle.9 Multiple studies have used a modified cut off when using the commercial cELISA in cattle and therefore there is no consensus on the method and hence sensitivity of the commercial kit.2,3,9 Furthermore, the sensitivity of this test varies over time as the antigens are released intermittently.5,7 An advantage of the method over the antibody ELISA is that worm burden can be differentiated pre- and post- anthelmintic treatment.2 Unfortunately, a fecal sample was not submitted for testing in this case.
Finding F. hepatica on liver necropsy is considered gold-standard however this method is not available for living animals. Estimated sensitivity and specificity are 99% and 98%, respectively.7 These values are much higher compared to liver inspections at abattoirs which were estimated to have a sensitivity and specificity of 68% and 88%, respectively.7 Liver histopathology can support a diagnosis of liver fluke, although sections often do not contain trematodes themselves. In this case, the size of the fluke and lack of gonad fits with the life cycle as juvenile flukes migrate through the liver parenchyma to the bile ducts where they become sexually mature.
Typical hepatic lesions in acute infection are characterized by hemorrhagic tortuous tracts with an element of coagulative necrosis and occasional inclusion of young flukes. As the disease progresses, these lesions are infiltrated by eosinophils followed by macrophages and giant cells which clear away the debris before healing occurs by formation of granulation tissue and fibrosis. Studies have shown that immature F. hepatica enters the liver parenchyma at 0.2 mm long and in cattle are approximately 1.5-3 mm long 21 days post-infection.8,12 Most juvenile flukes migrate to the bile ducts; however, some encyst in a fibrous capsule within the parenchyma.8 Mature flukes grow to approximately 2.5 cm long and reside in the large bile ducts and cause cholangitis. Biliary hyperplasia is a result of irritation by the flukes and biliary stasis. In this case, there is evidence of both acute and chronic fluke infection, with the presence of immature F. hepatica and migration tracts, and biliary hyperplasia and portal fibrosis, respectively. This suggests that this animal has been previously infected, with current reinfection.
Contributing Institution:
Elizabeth Macarthur Agricultural Institute
Woodbridge Rd
Menangle, NSW, Australia
www.regional.nsw.gov.au
JPC Diagnosis:
Liver: Fibrosis, portal, bridging, diffuse, moderate to severe, with biliary hyperplasia, parasite migration tracts, and larval trematode.
JPC Comment:
Fasciola hepatica has a broad geographic range and causes estimated annual losses of US $3.5 billion in the global livestock industry; beyond livestock, many species of mammals are affected with approximately 170 million humans worldwide at risk of infection.4 Many mammals serve as definitive hosts for the fluke, including cattle, sheep, buffalo, and humans.
F. hepatica has a rather complicated life cycle which begins when non-embryonated eggs are shed in the feces of an infected definitive host.1,10 Eggs become embryonated and hatch in water, followed by release of miracidia which then penetrate an aquatic snail which serves as the intermediate host.1,10 Development continues within the intermediate host until the free-swimming cercarial form is released into the environment.1,10 Cercariae encyst on plants and form metacercariae which are then ingested by a human or animal host.1,10 As noted by the contributor, once inside the host the metacercariae migrate through the duodenal wall, the peritoneal cavity, and then through the parenchyma of the liver for six to seven weeks until they reach the bile duct, where they mature into adults.1,10 Once a patent infection is established, flukes are prodigious egg layers, with a single adult fluke contaminating the environment with 20,000-50,000 eggs per day over a long time period.1 In cattle, egg production eventually begins to decline as the animal develops resistance to chronic infection.
Acute fascioliasis may occur following heavy intake of metacercariae, typically when livestock are grazed on heavily contaminated, wet ground.1 Clinical signs may be absent, or may include abdominal pain and/or jaundice. Death is usually the result of liver hemorrhage secondary to the immature fluke’s migration through the hepatic parenchyma.1 Subacute disease is heralded by jaundice, ill thrift, and anemia secondary to extensive migration-related liver damage which results in death in 8-10 weeks.1
Chronic fascioliasis is the most common form of the disease in cattle, sheep, goats, horses, and pigs, and develops when the flukes reach the bile ducts, begin ingesting blood, and cause anemia, chronic inflammation, and enlargement of the bile ducts.1 The disease is insidious, with increasing severity of anemia, decreasing appetite, reluctance to move, and, in some animals, submandibular edema (“bottle jaw”).1
A complication of fluke infection is Black disease, an acute and fatal liver disease that develops in fluke-infested sheep and cattle. Black disease is caused when fluke migration-induce liver damage provides a suitably anaerobic environment for the germination of Clostridium novyi type B spores within the liver. C. novyi multiplies in areas of hepatic necrosis and elaborates a necrotizing alpha toxin which causes more tissue damage, leading to a vicious cycle of bacterial proliferation and toxin elaboration. The disease, also called infectious necrotic hepatitis, causes acute death primarily in sheep, sometimes in cattle, and rarely in pigs and horses.1 Control is largely through reduction of fluke or intermediate host (snail) numbers, though a C. novyi toxoid is available for use to control outbreaks.
This week’s conference was a double-feature, with Dr. Paul Stromberg, Professor Emeritus at The Ohio State University College of Veterinary Medicine, and Dr. Don Meuten, Professor Emeritus at North Carolina State University College of Veterinary Medicine, sharing moderator duties. Dr. Stromberg began discussion of this case by noting the loss of tissue architecture, the “alarmed” hepatocytes, and the abundant inflammation and necrosis present within the liver. These acute changes are present against a background of severe fibrosis and chronic inflammation.
The moderator noted that the histologic features of the trematode, particularly the lack of a reproductive tract, the trematode as a juvenile and this, along with the gross findings of adult trematodes in the biliary tree, indicate that this animal is likely living in a contaminated environment and subject to recurrent bouts of infection, explaining the acute-on-chronic nature of the observed lesions. The moderator noted the dilated sinusoids and attenuated hepatocytes and speculated that these changes result from impeded hepatic circulation due to the massive hepatic fibrosis appreciated in section.
References:
- Boray JC, Love S. Liver fluke disease in sheep and cattle. NSW Department of Primary Industries. 2017.
- Brockwell YM, Spithill TW, Anderson GR, Grillo V, Sangster NC. Comparative kinetics of serological and coproantigen ELISA and faecal egg count in cattle experimentally infected with Fasciola hepatica and following treatment with triclabendazole. Vet Parasitol. 2013;196 (3-4):417-426.
- Charlier J, De Meulemeester L, Claerebout E, Williams D, Vercruysse J. Qualitative and quantitative evaluation of coprological and serological techniques for the diagnosis of fasciolosis in cattle. Vet Parasitol. 2008;153(1-2):44-51.
- Garza-Cuartero L, Garcia-Campos A, Zintl A, et. al. The worm turns: trematodes steering the course of co-infections. Vet Pathol. 2014;51(2):385-392.
- George SD, George AJ, Rolfe PF, Emery DL. Comparative assessment of faecal diagnostics for detection and predictive modelling of endemic Fasciola hepatica infection in sheep and cattle on Australian farms. Vet Parasitol. 2019;276:S100001.
- Martínez-Sernández V, Orbegozo-Medina RA, González-Warleta M, Mezo M, Ubeira FM. Rapid enhanced MM3-COPRO ELISA for detection of Fasciola coproantigens. PLoS Negl Trop Dis. 2016;10(7):e0004872.
- Mazeri S, Sargison N, Kelly RF, Bronsvoort BM deC., Handel I. Evaluation of the performance of five diagnostic tests for Fasciola hepatica infection in naturally infected cattle using a bayesian no gold standard approach. Yu X, ed. PLoS One. 2016;11(8):e0161621.
- Moazeni M, Ahmadi A. Controversial aspects of the life cycle of Fasciola hepatica. Exp Parasitol. 2016;169:81-89.
- Palmer D, Lyon J, Palmer M, Forshaw D. Evaluation of a copro-antigen ELISA to detect Fasciola hepatica infection in sheep, cattle and horses. Aust Vet J. 2014; 92(9):357-361.
- Pecararo HL, Stenger BLS, Rice LE, Webb BT. Gross and histologic description of trematodiasis in fetal and neonatal beef calves in North Dakota and Minnesota. J Vet Diagn Invest. 2022;34(5):870-873.
- Reichel MP, Vanhoff K, Baxter B. Performance characteristics of an enzyme-linked immunosorbent assay performed in milk for the detection of liver fluke (Fasciola hepatica) infection in cattle. Vet Parasitol. 2005;129(1-2):61-66.
- Ross JG, Todd JR, Dow C. Single experimental infections of calves with the liver fluke, Fasciola hepatica (Linnaeus 1758). J Comp Pathol. 1966;76(1):67-81.
- Salimi-Bejestani MR, McGarry JW, Felstead S, Ortiz P, Akca A, Williams DJL. Development of an antibody-detection ELISA for Fasciola hepatica and its evaluation against a commercially available test. Res Vet Sci. 2005;78(2):177-181.
- Statham JME. Control of liver fluke: an emerging issue in terms of veterinary residues. Vet Rec. 2015;177(20):519-521.