Signalment:
Gross Description:
Morphologic Diagnosis:
Lab Results:
Condition:
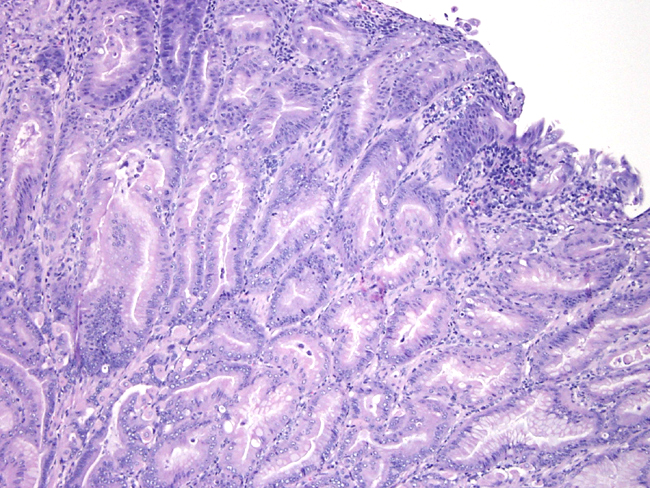
Contributor Comment:
The insulin gastrin (INS-GAS) mouse is a transgenic mouse bearing the transgene that overexpresses amidated gastrin, the main biologically active form of gastrin. The transgene targets expression of human gastrin to the pancreatic islets. In adult mammals, gastrin is expressed mainly in the astral G cells of the stomach where it controls the acid secretion and stimulates mucosal proliferation. The INS-GAS mice spontaneously develop gastric cancer, but this requires the virtual lifetime of the animal (1-2 years). However, when male INS-GAS mice are infected with H. pylori, they uniformly developed atrophy, intestinal metaplasia, and dysplasia by 6 weeks and carcinoma by 24 weeks.Â
Gastric carcinoma is the most common of malignant tumors of the stomach in people. It is the second most common tumor in the world. It exhibits a male-to-female ration of about 2:1. The major factors thought to affect the genesis of gastric cancer include environmental factors, host factors and genetic factors. One environmental factor, infection by H. pylori, present in most cases of intestinal-type carcinoma. Chronic infection with H. pylori generally increases the risk for developing gastric carcinoma by five-to six-fold. The bacterial infection causes chronic gastritis, followed by atrophy, intestinal metaplasia, dysplasia and carcinoma. One host determinant that may influence the development of gastric cancer is gastrin. Hypergastrinemia occurs early in the course of human H. pylori infection, precedes the development of atrophic gastritis, and often resolves after eradication. In addition, in vitro gastrin stimulates gastric epithelial cell proliferation.
In addition to host factors, bacterial determinants also contribute to gastric carcinogenesis. One virulence-associated H. pylori constituent is the CagA (cytotoxin associated gene A) pathogenicity island, which is present in approximately 60% of United States strains, and carriage of a Cag+/- strain augments the risk for atrophic gastritis and distal gastric adenocarcinoma compared with that incurred by Cag -/- strains. Several cag genes encode products that possess homology to components of type IV secretion systems, and, following H. pylori adherence to epithelial cells in vitro, the product of the terminal gene in the island (CagA) is translocated into the host cell, in which it undergoes Src-dependent phosphorylation and activates a phosphatase (SHP-2), leading to cellular morphologic changes. Loss of CagE temporally retards but does not abrogate pathologic progression.
All experiments were approved by the Committee on Animal care at Massachusetts Institute of Technology.
JPC Diagnosis:
2. Duodenum and pancreas: No significant lesions
Conference Comment:
Conference participants reviewed the primary cell types and functions of cells located within the stomach, such as parietal cells, chief cells, G cells, D cells, enterochromaffin-like cells, and mucous neck cells.
In the INS-GAS mouse, human heptadecapeptide gastrin is produced by the islet _ cells and secreted into circulation. This gastrin release in turn causes an initial stimulation of gastric acid secretion (2-3 months of age), increasing the number of parietal and enterochromaffin-like cells. However, mice older than 5 months have a decline in acid secretion until 20 months of age when there is virtually no secretion at all, which coincides with decreasing numbers of parietal and enterochromaffin-like cells.8
Helicobacter spp. are Gram-negative spirochetes, approximately 3_m in length with 4-6 flagellae. Several species may contain ureases, catalases, oxidases, proteases, and phospholipases. Helicobacter spp. have been implicated in gastric lymphoma and carcinoma in ferrets and humans. Helicobacter hepaticus can cause an acute focal, non-suppurative necrotizing hepatitis in mice.
In this case, despite experimental inoculation, no Helicobacter sp. were noted with Warthin-Starry or Steiners stains performed at AFIP.
Helicobacter sp. in various animal species:
| Mouse5 | H. bilis, H. hepaticus, H. muridarum, H. rodentium, H. typhlonius, H. ganmani, H. rappini, H. mastomyrinus, H. muricola (Korean wild mice) |
| Rat5 | H. bilis, H. muridarum, H. rodentium, H. trogontum, H. typhlonius |
| Ferret2 | H. mustelae |
| Hamster5,6 | H. aurati, H. cineadi, H. mesocricetorum, H. cholecystus |
| Gerbil5 | H. hepaticus, H. bilis |
| Dog2 | H. felis, H. heilmannii |
| Cat2 | H. felis, H. pylori, H. heilmannii |
| Pig2 | H. heilmannii |
References:
2. Dubois A: Animal models of Helicobacter infection. Lab Anim Sci 48:596-603, 1998
3. Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, Peek RM: Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology 124:1879-1890, 2003
4. Liu C, Crawford JM:The gastrointestinal tract. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas, AK, Fausto N, 7th ed., pp. 822- 824. Elsevier Saunders, Philadelphia, PA, 1999
5. Owen RJ: Helicobacter species classification and identification. Br Med Bull 54:17-30, 1998
6. Percy DH, Barthold SW: Mouse. In: Pathology of Laboratory Rodents and Rabbits, 3rd ed., pp. 58-61, 140, 190, 211. Blackwell Publishing, Ames, IA, 2007
7. Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox, JG: Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res 65:10709-10715, 2005
8. Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychodhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG: Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118:36-47, 2000
9. Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ: Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98:1918-1929, 1996