Signalment:
11-year-old, castrated male, Labrador retriever, (
Canis familiaris).The patient presented six months prior to euthanasia for
evaluation of a cardiac arrhythmia and a several month history of intermittent
coughing which had become more severe in the two days before initial
presentation. An intermittent split P wave was noted on the ECG. Thoracic radiographs
revealed a markedly enlarged heart, right atrial enlargement, enlarged
pulmonary vessels, and a diffuse broncho-alveolar pattern. Echocardiography
showed a space-occupying mass in the left atrium with mitral and tricuspid
valve regurgitation. The patient was discharged with furosemide and returned six
months later with exercise intolerance and respiratory distress. At that time
due to the patients declining condition the owner elected euthanasia.
Gross Description:
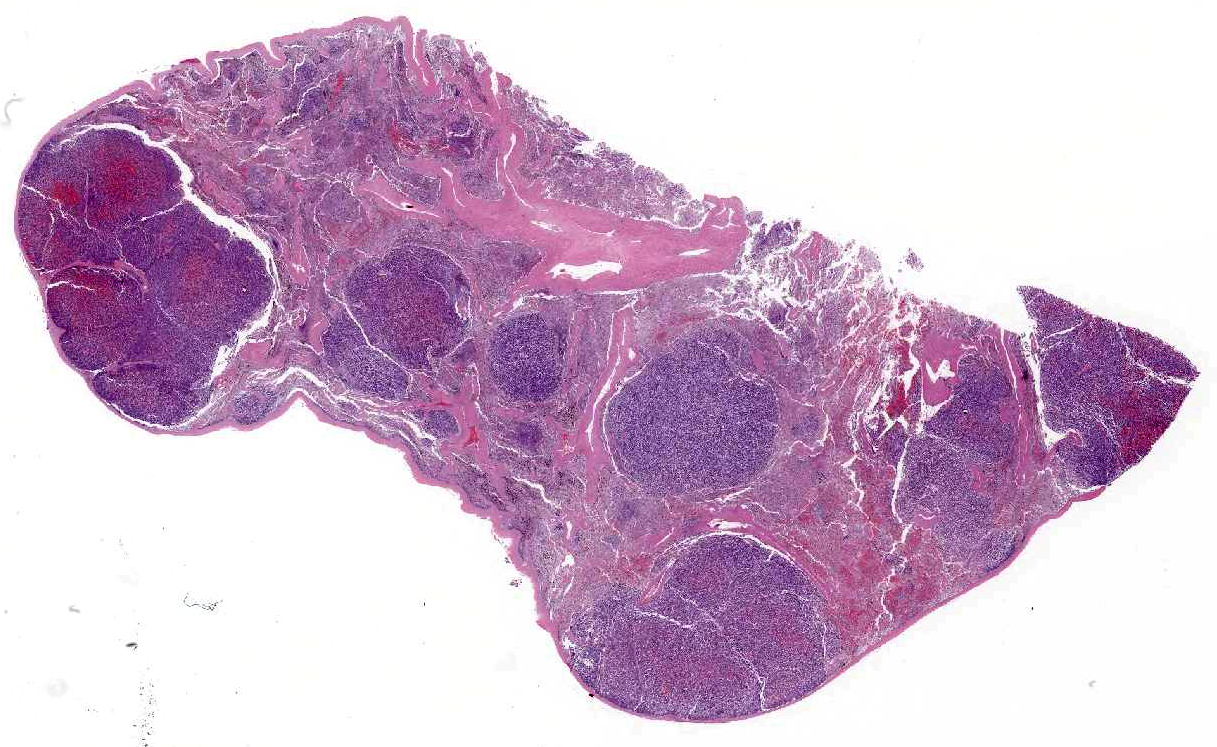
A 5 x 6 x 8 cm, firm, multinodular mass is present on the
proximocaudal aspect of the heart base, resting on the left atrium. On cross
section, the mass is highly vascular and has a mottled red-to-brown mosaic
pattern. The mass surrounds the pulmonary arteries, compressing the left
pulmonary artery, but it is still patent. The mass is proximal to the
pulmonary veins that are not compressed. Tumor invasion into the left atrial
lumen is characterized by numerous nodular small projections, up to 0.5 cm,
covered by intact endocardium. Left atrial luminal size is decreased due to
compression by the mass. Mild endocardiosis of the mitral and tricuspid valves
is evident. The right ventricle is distended and dilated in addition to an
overall enlarged heart.
The
abdomen contains 4 liters of sero-sanguinous clear fluid, with another 2 liters
of clear creamy yellowish fluid in the thoracic cavity. The spleen has multiple
firm white and hemorrhagic masses ranging from 1 mm to 3 cm in size. The
consistencies of the splenic masses are similar to the heart mass. The left
anterior lung is very pale, soft, and slightly decreased in size due to
compression by the left atrial mass. Mediastinal lymph nodes are enlarged with
effacement of normal architecture.
Histopathologic Description:
Spleen:
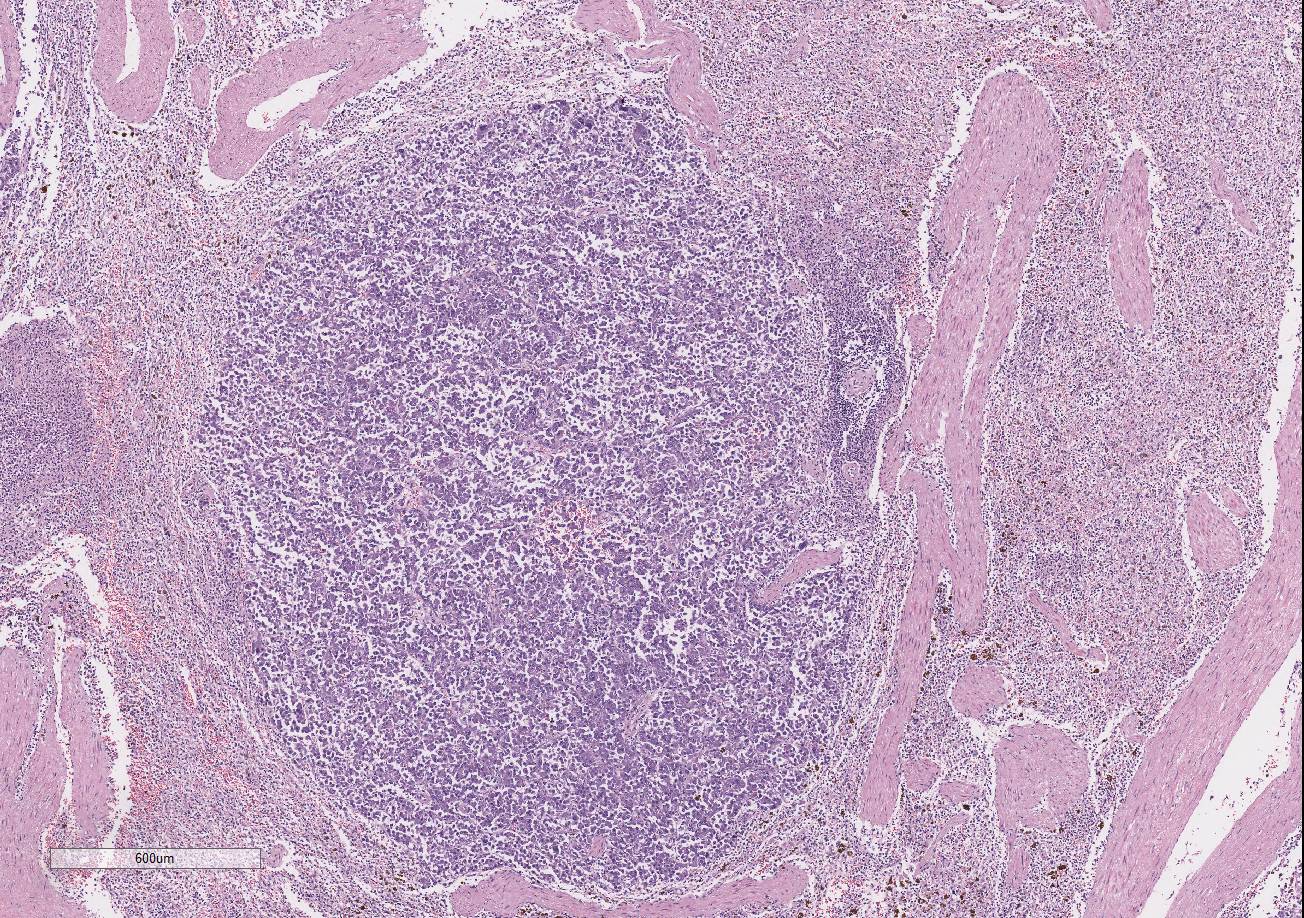
There are multiple variably-sized solid nodules
effacing and replacing the splenic parenchyma. The neoplastic nodules are well
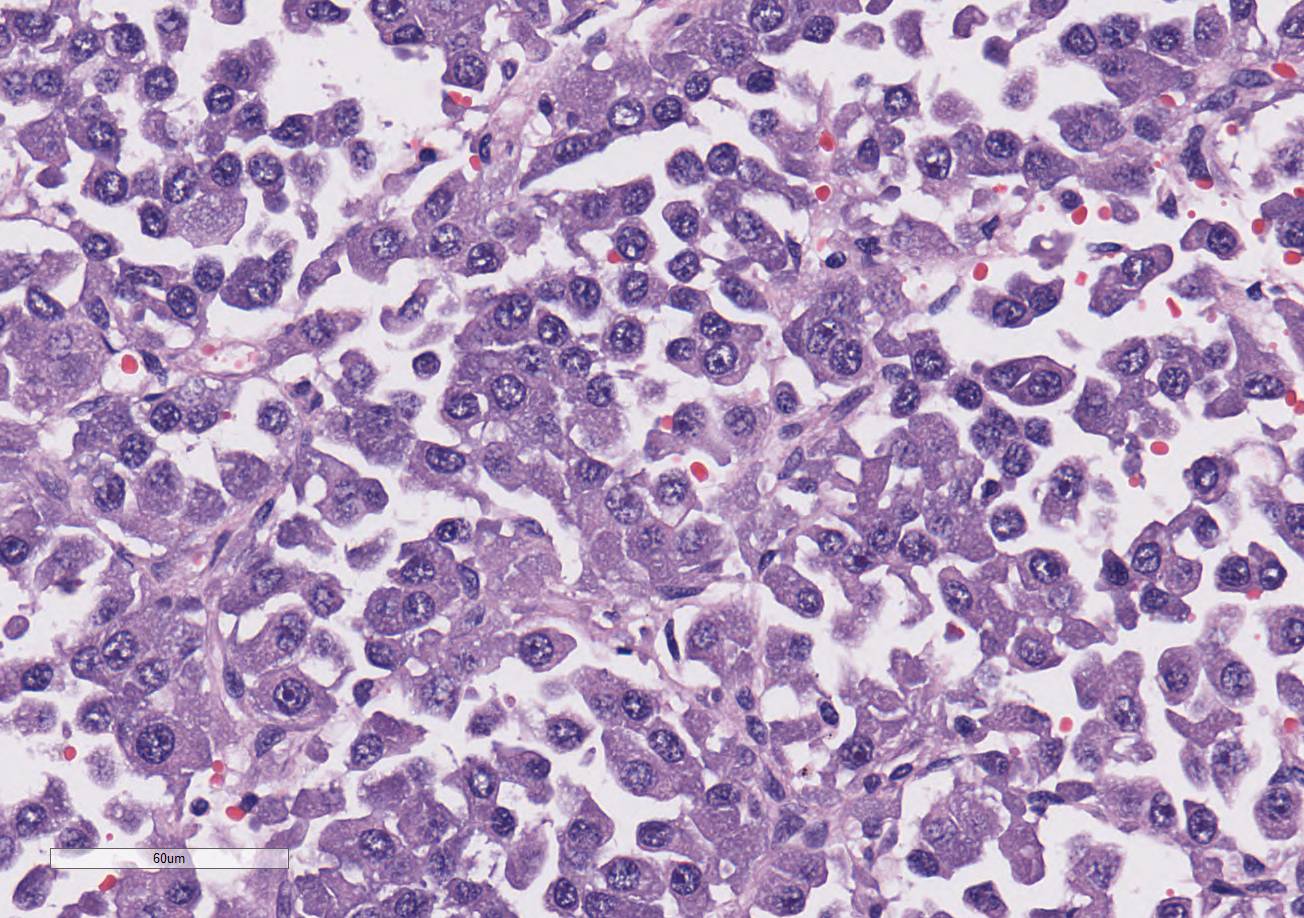
demarcated and expansile. They are composed of large islands of polygonal cells
separated by large connective tissue branches and supported by fine fibro-vascular
stroma that subdivide the cells in small ribbons. The tumor cells are cuboidal
to polyhedral in shape with a round nucleus and a moderate amount of granular
lightly amphophilic cytoplasm. The cells are aligned along the small
capillaries forming small packets where the cells show polarity with the
cytoplasm towards the vessels. Mitotic activity is 11 mitotic figures in 10
hpf. There are areas of marked aniso-karyosis and occasional megalokaryosis
(polymorphism) with prominent nucleoli. The large cells show a round
eosinophilic prominent nucleolus.
The adjacent splenic parenchyma is compressed and attenuated.
There are small multiple aggregates of macrophages filled with large granulated
golden pigment (hemosiderin- previous hemorrhages). The smooth muscle
trabeculae are closer than expected due to collapse of the parenchyma with very
few follicles.
Morphologic Diagnosis:
1. Heart: Chemodectoma
2. Heart: Mild diffuse mitral and tricuspid valve myxomatous
degeneration
3. Spleen: Chemodectoma, metastasis
4. Liver: Chronic severe congestive hepatopathy
5. Lymph Nodes: Chemodectoma, metastasis
Lab Results:
Upon initial presentation, NOVA results are as follows:
Glucose: 118 mg/dL (60-115)
Ca++: 1.35 mmol/L (2.2-3.0)
Mg++: 0.49 mmol/L (1.5-2.5)
PCO2: 26.1 mmHg (34-40)
PO2: 49.1 mmHg (85-100)
PO2: 49.1 mmHg (85-100)
SO2: 85.4 % (>90)
BEecf: -7.3 mmol/L ([0]-[+6])
nCa: 1.37 mmol/L (1.13-1.33)
nMg: 0.50 mmol/L (0.26-0.41)
Condition:
Neuroendocrine carcinoma
Contributor Comment:
Spleen
is the only tissue submitted. Chemodectomas are primary neuroendocrine tumors
arising most commonly from the chemoreceptor organs of the aortic and carotid
bodies.
1,2,4,12 Other chemoreceptors that rarely develop neoplasia
include the nodose ganglion of the vagus nerve, ciliary ganglion in the orbit,
the pancreas, and the glomus jugulare along the recurrent branch of the
glossopharyngeal nerve.
11 They are synonymous with heart base tumors
or non-chromaffin, extra-adrenal, paragangliomas in veterinary medicine.
3,4
Chemodectomas
are uncommon neoplasms most often described in canines where they have an
incidence of 0.19%.
14 Chemo-dectomas are the second most common
cardiac tumor, behind hemangiosarcomas, representing 8% of cardiac tumors.
14
Contrary to humans, aortic body tumors are the more common form of chemodectoma
in animals with carotid body tumors being less common and more malignant.
4
In dogs, aortic body tumors are encountered up to four times more frequently
than carotid body tumors.
12 The aortic body is located adjacent to
the adventitia of the aortic arch at the bifurcation of the subclavian artery
while the carotid body is located at the bifurcation of the common carotid
artery.
1,4,14 Paren-chymal cells of neuroectodermal origin and
sustentacular or stellate cells are the primary cell types that make up the
chemoreceptor organs.
4,11 The function of the aortic and carotid
bodies is to sense fluctuations in the carbon dioxide, pH, and oxygen tension
in blood, which helps to regulate respiration and circulation.
4,7
The aortic and carotid bodies can increase heart rate and elevate arterial
blood pressure through the sympathetic nervous system and alter the depth, minute
volume, and rate of respiration through the parasympathetic nervous system.
4
The chemoreceptor system is considered part of the parasympathetic nervous
system, as it does not secrete catecholamines; however, the presence of secretory
granules in the cytoplasm of the glomus cells, the functional cells of
chemoreceptors, is inconsistent with this finding.
1,11,12
Due
to the lack of catecholamine production, the tumors clinical manifestations
are the result of being a space-occupying lesion. Smaller adenomas may go
undetected, as they can be too small to cause clinical signs, while larger
adenomas may press on the atria or displace the trachea and partially surround
the major vessels at the base of the heart. Aortic body tumors can also be
locally invasive and invade the lumen of the surrounding great vessels or heart
chambers hindering blood flow.
4 Common clinical manifestation of
aortic body tumors include ascites, pulmonary edema, nutmeg liver, hemothorax,
hemopericardium, anasarca, dyspnea, cyanosis, splenomegaly, and arrhythmias.
6,10,12,15
Many of these clinical manifestations are consistent with right-sided
congestive heart failure brought on by the tumor acting as a space occupying
lesion or by local invasion of vessels resulting in the obstruction of blood
flow.
15 Another way aortic body tumors cause lesions is through
metastasis to other parts of the body. It is rare for an aortic body tumor to
metastasize, with the most common locations being the lungs and liver.
4
However, other locations have been identified including lymph nodes,
myocardium, kidney, adrenal gland, bladder, spleen, and even bone.
4,5,7,12,14
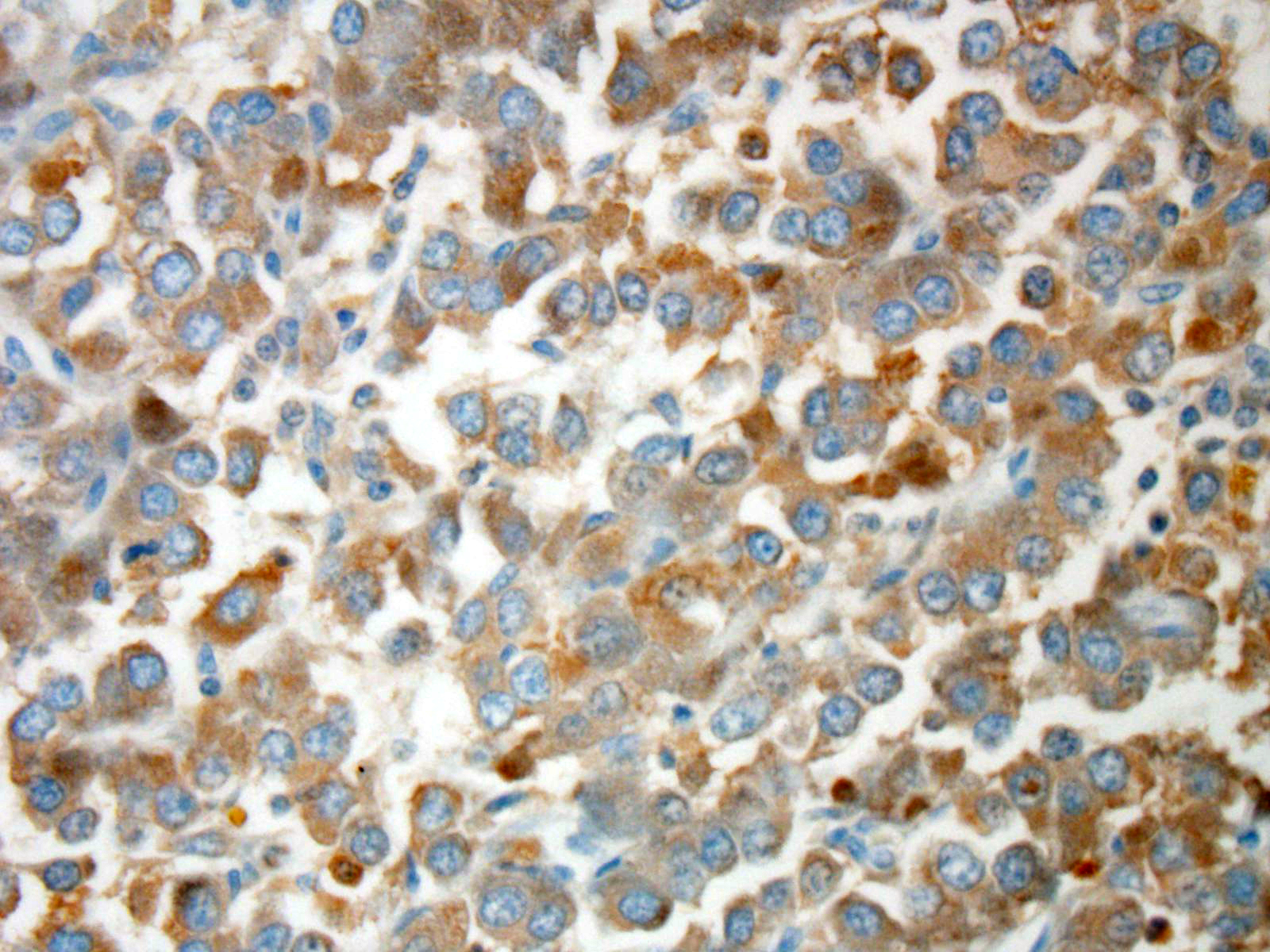
Immuno-histochemistry has been valuable to definitively confirm the diagnosis
of chemodectomas.
8,12,15
JPC Diagnosis:
Spleen: Neuroendocrine carcinoma, metastatic, Labrador retriever,
Canis
familiaris.
Conference Comment:
The
contributor provides an excellent summary of the key features of chemodectomas
in dogs. As is the tradition at the Joint Pathology Center, conference
participants are not provided the full signalment, history, gross necropsy
findings, or results of additional histo-chemical and immunohistochemical
stains prior to the conference. Conference participants described nests and
packets of neoplastic polygonal cells with small, round, hypochromatic nuclei
separated and supported by a delicate fibrovascular stroma, and aptly
determined the neoplasm to be of neuroendocrine origin; however, without the additional
clinicopathologic information, the site of origin could not be definitively determined
from histopathologic evaluation alone. As a result, participants
discussed the most likely sites of origin for neuro-endocrine chemoreceptor
paragangliomas (also known as chemodectomas and glomus tumors) in animals.
Chemoreceptor organs are present in several sites of the body,
such as the cartotid body, aortic body, nodose ganglion of the vagus nerve,
ciliary ganglion of the orbit, pancreas, jugular vein, middle ear, and glomus
jugulare near the glossopharyngeal nerve.
13 As mentioned by the
contributor, they are all highly sensitive to changes in blood pH, oxygen
tension, and temperature, and can rapidly change respiratory and heart rate via
the autonomic nervous system.
13 Metastasis occurs in about 1/3
rd
of cases of chemodectomas arising from the carotid body, and multicentric
neoplastic trans-formation has been frequently reported in brachiocephalic dogs,
possibly because of breed-associated anatomic malformations in the upper
respiratory tract resulting in chronic hypoxia.
13
Neoplastic cells in
neuroendocrine tumors contain variable numbers of cytoplasmic secretory
granules, best visualized by electron microscopy. Additionally, the number of
granules is used to distinguish adenomas, which contain more numerous granules,
from carcinomas. In addition, neoplastic cells typically will be immunopositive
for chromogranin-A, neuron-specific enolase (NSE), synapto-physin, and S100
protein. Prior to the conference, the JPC performed tissue immunohistochemistry
for chromogranin-A, NSE, and synaptophysin. Unfortunately neoplastic cells were
immunonegative for all three stains; however, participants speculated the
results may be due to suboptimal tissue fixation.
13
References:
1. Aupperle H, März I, Ellenberger C, Buschatz S, et al. Primary
and secondary heart tumours in dogs and cats.
J Comp Pathol. 2007; 136:18-26.
2. Brown PJ, Rema A, Gartner F. Immunohistochemical
characteristics of canine aortic and carotid body tumours.
J Vet Med A Physiol Pathol Clin Med.
2003;50:140-144.
3. Balaguer L, Romano J, Neito JM, Vidal S, Alverez
C. Incidental finding of a chemodectoma in a dog:
Differential diagnosis.
J Vet Diagn Invest. 1990; 2:339-341.
4. Capen CC: Endocrine glands.
In: Maxie MG, ed. Jubb, Kennedy,
and Palmer's Pathology of Domestic Animals. 5th ed. Vol 3. St. Louis, MO:
Elsevier; 2007:425-428.
5. Eriksson, Malin. "Aortic Body Tumors in Dogs."
(2011). http://stud.epsilon.slu.se/2545/1/Eriksson_m_110502.pdf
6. Ehrhart N, Ehrhart, EJ, Willis J, Sisson D, Constable P, Greenfield
C, Manfra Maretta S,
Hintermeister J. Analysis of factors affecting survival in dogs with aortic
body tumors.
Vet Surg.
2007; 31(1):44-48.
7. Johnson KH. Aortic body tumors in dogs.
J Am Vet Med Assoc. 1968; 152(2):154.
8. Khodakaram-Tafti A, Shirian S, Shekarforoush SS, Fariman H,
Daneshbod Y. Aortic body chemodectoma in a cow: Clinical, morphopathological,
and immunohistochemical study.
Comp
Clin Pathol. 2011;20(6):677-679.
9. McManus BM, Allard MF, Yanagawa R. Hemodynamic disorders. In:
Rubins Pathology: Clinicopathologic Foundations of Medicine. ed. Rubin
R, Strayer DS. 5
th ed. Philadelphia, PA: Wolters-Kluwer;
2008:231-232.
10. Noszczyk-Nowak, Agnieszka, Nowak M, Paslawska U, Atamaniuk W,
Nicpon J. Cases with manifestation of chemodectoma diagnosed in dogs in
Department of Internal diseases with Horses, Dogs and Cats Clinic, veterinary
medicine faculty, University of Environmental and Life Sciences, Wroclaw,
Poland."
Acta Veterinaria
Scandinavica. 2010; 52:35.
11. Owen TJ, Bruyette DS, Layton CE. Chemodectoma in dogs.
Comp
Cont Educ Pract. 1996;18:253265.
12. Paltrinieri S, Riccaboni P, Rondena M, Giudice C. Pathologic and immunohistochemical findings in a
feline aortic body tumor.
Vet Pathol.
2004; 41:195-198.
13. Rosol TJ, Grone A. Endocrine glands. In: Maxie MG, ed.
Jubb,
Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 3. St.
Louis, MO: Elsevier; 2016:354-357.
14. Ware WA, Hopper DL. Cardiac tumors in dogs: 19821995.
J
Vet Intern Med
. 1995; 13: 95-103.
15. Willis R, Williams AE, Schwarz T, Paterson C, Wotton PR. Aortic
body chemodectoma causing pulmonary oedema in a cat. J
Small Anim Pract.
2001;
42:20-23.